US Pharm. 2024;49(2):30-37.
ABSTRACT: Stroke is associated with significant morbidity and mortality. Over 60% of cases are due to ischemic stroke, which is further divided into five subtypes. Antithrombotic therapy, i.e., antiplatelet or anticoagulant therapy, is a mainstay in the primary and secondary prevention of ischemic stroke. Recommendations for the use of antithrombotics vary by numerous factors, including stroke type. Antithrombotic therapy places patients at increased risk of bleeding, and pharmacists can ensure the safe and optimial use of these agents by educating patients on their proper use and monitoring patients for the occurrence of adverse drug events.
Stroke is a leading cause of morbidity and mortality in the United States. According to the CDC, in 2021, one in six deaths from cardiovascular disease (CVD) was due to stroke. It is estimated that more than 795,000 persons experience a stroke per year, with about 610,000 being a first or new stroke and about 185,000 recurrent strokes.1
Stroke rate varies by age, geographic location, and race. Although stroke is more common in older adults, according to data from 2014, 38% of stroke hospitalizations were for patients aged <65 years. The risk of first stroke is nearly doubled in non-Hispanic blacks compared with whites.1
The death rate for stroke increased from 38.8 per 100,000 in 2020 to 41.1 per 100,000 in 2021, after declining from 2001 to 2013.1,2 Stroke mortality was highest in the South and lowest in the Northeast.3
Types of Stroke
There are two major types of stroke: ischemic and hemorrhagic. Ischemic stroke occurs when thrombi block the blood vessels in the brain. Blockage may also be due to a buildup of atherosclerotic plaque. Hemorrhagic stroke occurs when an artery in the brain leaks or ruptures, resulting in increased intracranial pressure.4 Hemorrhagic strokes are due to intracranial or subarachnoid hemorrhages.5,6
Ischemic stroke accounts for about 62% of stroke cases, whereas intracranial and subarachnoid hemorrhages make up 28% and 10% of stroke cases, respectively.6
Primary Versus Secondary Stroke Prevention
Primary prevention refers to avoiding the first stroke event, whereas secondary prevention pertains to preventing recurrent stroke.7
Up to 90% of all strokes are considered preventable and are associated with one of 10 risk factors, including a history of hypertension or blood pressure (BP) >140/90 (1/3 of strokes), lack of physical activity, apolipoprotein B/A1 ratio, diet, waist-to-hip ratio, psychosocial factors, smoking, cardiac causes (e.g., atrial fibrillation [AFib], myocardial infarction, rheumatic heart disease, peripheral arterial disease), alcohol intake, and history of diabetes or hemoglobin A1c >6.5. Controlling these risk factors can reduce stroke risk.7
Following a stroke, it is important to still address these primary prevention measures in addition to specific secondary preventative measures based on the type of stroke.
Ischemic Stroke Types and Pathophysiology
The TOAST (Trial of Org 10172 in Acute Stroke Treatment) classification categorizes ischemic strokes based on their clinical features and the results of ancillary diagnostic studies into five subtypes, which include cardioembolic stroke (CES), cryptogenic stroke, stroke from large artery atherosclerosis (SLAA), stroke from small vessel disease (SSVD), and stroke of other determined etiology (SODE).8
Cardiometabolic Stroke: CES accounts for about 27% of ischemic strokes.6,9 A CES results from obstruction of cerebral blood flow and is associated with endothelial injury, stasis, and hypercoagulability. It can be due to atrial disease (e.g., arrhythmias such as AFib or sick sinus syndrome), structural disease, valvular heart disease, structural and functional ventricular disorders, or myocardial infarction.6,9
Cryptogenic Stroke: Cryptogenic stroke, which refers to ischemic stroke of unknown origin, is the most common form of ischemic stroke, accounting for over one-third (35%) of cases. Embolic strokes of undetermined source (ESUS) account for about one-half of cryptogenic strokes and may be due to atrial cardiopathy, nonstenotic plaques, hypercoagulable states, cancer, prothrombotic states, aortic arch atherosclerosis, occult AFib, genetic factors, or paradoxical embolism from the venous circulation through a patent foramen ovale (PFO).6,10,11
Stroke From Large Artery Atherosclerosis: SLAA is not as common as other forms of ischemic stroke and accounts for only about 13% of ischemic strokes.6,12 In SLAA, there is stenosis of >50% or complete occlusion of intracranial (anterior and middle cerebral and basilar) or extracranial arteries (carotid and vertebral) due to atheromatous plaques.6
Stroke From Small Vessel Disease: SSVD accounts for almost one-quarter (23%) of ischemic stroke. It involves small subcortical infarcts due to occlusion of small, deep perforating arterioles. Risk factors for strokes from SSVD include hypertension and lipohyalinosis, i.e., the deposition of eosinophilic material into the connective tissue of the wall of deep-penetrating arteries.6,13,14
Stroke From Other Determined Etiology: SODE is the least common subtype of ischemic stroke, accounting for only about 2% of cases.6 This is a very heterogenous category. Among the conditions identified in this group are arterial dissection (accounting for ~40%); infectious or inflammatory diseases of extracranial and intracranial arteries; intrinsic diseases of the arterial wall; disorders of platelets and the hemostatic system; cancer-related coagulopathy; stroke associated with migraine and drugs (unspecified); hereditary disorders, including cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy; hypoperfusion syndromes due to pump failure, hyperviscosity, or altered vascular tone; and iatrogenic causes.15
Choosing an Appropriate Antithrombotic Agent
Factors to consider when choosing an antithrombotic agent include type of ischemic stroke, whether the patient is at risk of major bleeding, whether genetic polymorphism affects the antithrombotic, how long to treat, whether to use dual antiplatelet therapy (DAPT) or monotherapy, and potential for nonadherence. If antithrombotics are used for secondary prophylaxis, it is important to determine when to initiate antithrombotic therapy following the acute event and whether the patient is at risk of hemorrhagic transformation.16
Pharmacologic Therapy for Primary Stroke Prevention
Tools to Assess Primary Stroke Risk: Tools are available to assess both the risk of stroke and bleeding risk for anticoagulation in patients with AFib (see TABLE 1).17-25
Primary Prevention Based on Ischemic Stroke Type
In April 2022, the U.S. Preventive Services Task Force issued a statement on the use of aspirin for primary prevention of CVD, which includes stroke. It stated that the decision to initiate low-dose aspirin in those 40 to 59 years who have a >10% 10-year CVD risk should be individualized. Net benefit of the use of aspirin is small, and those at low risk of bleeding are most likely to benefit. However, it recommends against initiating low-dose aspirin for primary prevention in those aged >60 years as the risk for bleeding is increased.26
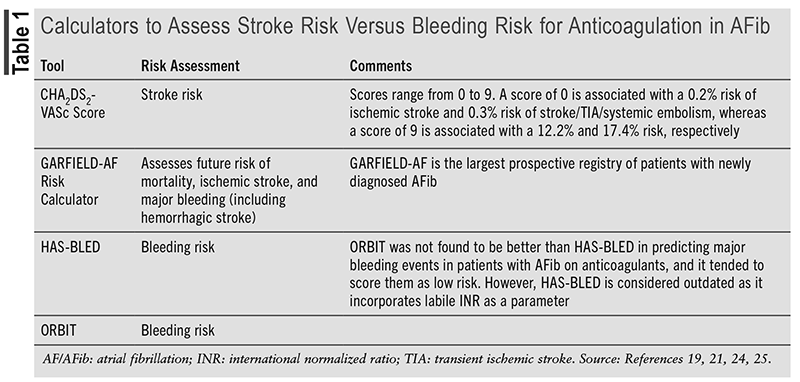
The ASCVD (atherosclerotic cardiovascular disease) Risk Calculator, which helps predict the 10-year risk for a first ASCVD event, can be found at https://clincalc.com/Cardiology/ASCVD/PooledCohort.aspx.
In January 2024, the American Heart Association released the Predicting Risk of cardiovascular disease EVENTs (PREVENT) tool. Use of the app is intended for primary prevention patients (those without ASCVD or heart failure) who are aged between 30 and 79 years.
The calculator provides 10-year risk estimates for individuals aged 30 to 79 years and 30-year risk estimates for individuals aged 30 to 59 years. The PREVENT Online Calculator can be found at https://professional.heart.org/en/guidelines-and-statements/prevent-calculator.
CES: The most common cause of CES is AFib. Anticoagulants are the drugs of choice for prevention as aspirin monotherapy is ineffective.6
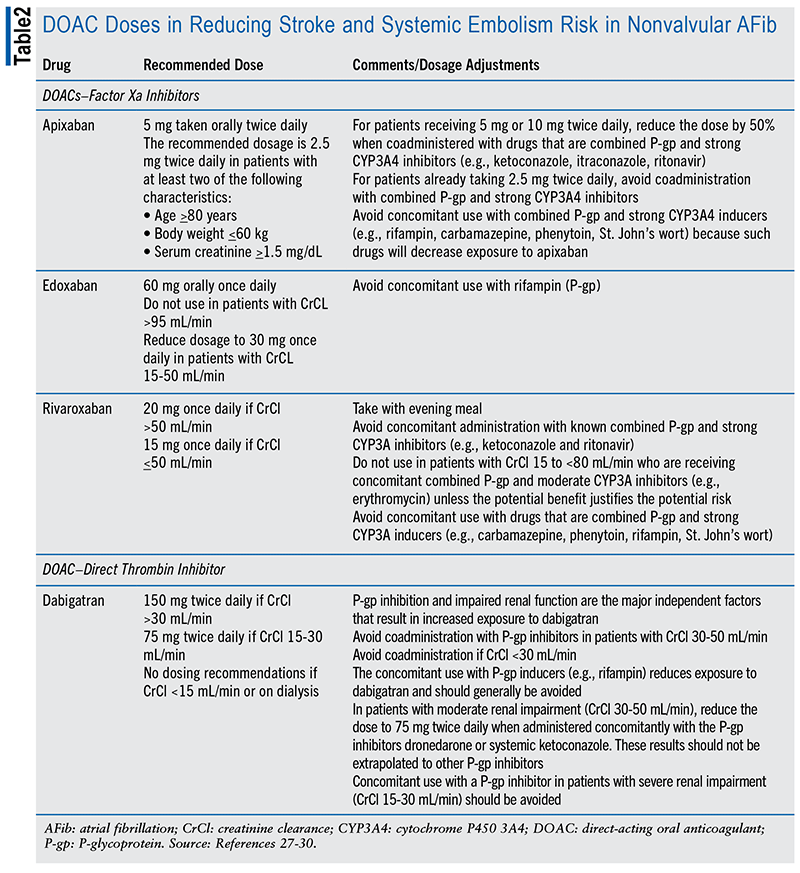
Warfarin has been the mainstay of therapy for CES, but due to the need for frequent monitoring of prothrombin time test-international normalized ratio (PT-INR) levels, potential for drug-drug interactions, and dietary restrictions, direct-acting oral anticoagulants (DOACs) have been increasingly favored in the management of AFib.6
DOACs, which include activated factor X inhibitors, e.g., rivaroxaban, apixaban, and edoxaban, and the activated factor II inhibitor dabigatran are at least as safe and effective as warfarin in preventing stroke in patients with AFib. Compared with warfarin in nonvalvular AFib, DOACs reduce ischemic stroke, hemorrhagic stroke, and all-cause mortality by 19%, 51%, and 10%, respectively. However, risk of gastrointestinal bleeding is increased by 25%, although apixaban’s risk may be lower.6,27-30 (See TABLE 2.)
DAPT with aspirin and clopidogrel is of limited benefit in AFib as it increases the risk of both major bleeding and intracranial hemorrhage.6
The 2023 ACC/AHA/ACCP/HRS guideline for the diagnosis and management of AFib recommends the following31:
• For patients with AFib and an estimated annual thromboembolic risk of >2% per year (which refers to a CHA2DS2-VASc score of >2 in men or >3 in women), anticoagulation is recommended.
• In patients with AFib without a history of moderate or severe rheumatic mitral stenosis or a mechanical heart valve (MHV) and who are candidates for anticoagulation, DOACs are recommended over warfarin.
• For patients with AFib and an estimated annual thromboembolic risk of >1% but <2% per year (i.e., similar to a CHA2DS2-VASc score of 1 in men and 2 in women), it is reasonable to consider anticoagulation.
• The guideline recommends against the use of aspirin alone or in combination with clopidogrel as an alternative to anticoagulation in patients with AFib who are candidates for anticoagulation and who do not have an indication for antiplatelet therapy, as the risk of harm is increased.
• For patients with AFib who do not have risk factors for stroke, aspirin monotherapy is not beneficial to prevent thromboembolic events.
The 2023 American Geriatrics Society Beers List of Potentially Inappropriate Medications states that warfarin use for the treatment of nonvalvular AFib is associated with a higher risk of major bleeding (particularly intracranial bleeding) and similar or lower effectiveness compared with DOACs. It states that DOACs are the preferred choice for anticoagulation for most patients with these conditions. It is recommended to not start warfarin for initial therapy unless alternatives are contraindicated or there are substantial barriers to their use. For older patients who have been using warfarin long-term, it may be reasonable to continue treatment, especially among those with well-controlled INRs (i.e., >70% time in the therapeutic range) and no adverse effects.32
Patients with MHVs should be managed on life-long warfarin therapy with a PT-INR in the range of 2.5 to 3.5. Oral anticoagulation should be initiated immediately after surgery since the risk of embolic events is highest in the first 6 months.6
Recommendations vary for bioprosthetic valves. For mitral or tricuspid bioprosthetic valves, warfarin therapy is administered for 3 months. For aortic surgical bioprosthetic valves, if a transcatheter aortic valve has been implanted, aspirin therapy is recommended if there are no other reasons for anticoagulation. DOACs are not inferior to warfarin for anticoagulation in patients with bioprosthetic valves.6
For infectious endocarditis, antithrombotic therapy is not warranted. The mainstay of treatment is antibiotic therapy.6
Cryptogenic Stroke: There are limited data on primary stroke prophylaxis of cryptogenic stroke. However, primary antithrombotic prophylaxis is not recommended in patients with paradoxical embolization through a PFO.6
According to the 2021 American Heart Association and American Stroke Association (AHA/ASA) secondary stroke prevention guideline, anticoagulation with a DOAC is preferred over warfarin in patients with ischemic stroke or transient ischemic attack (TIA), AFib, and cancer.33
SLAA: Although antiplatelet agents, including aspirin and clopidogrel, are routinely used for both primary and secondary prophylaxis of SLAA, the literature is plagued by methodological flaws, including heterogeneous patient populations, variations in inclusion criteria and stroke severity, timing from symptom onset, duration of antithrombotic treatment, and lack of power to determine stroke reduction, thereby limiting generalizability of these studies. The potential risk of bleeding from antiplatelet therapy needs to be weighed against the potential benefit of preventing an initial stroke.6,34
Aspirin should not be used for primary stroke prevention in persons with LAA who are at low risk of stroke or in diabetics who do not have any other risk factors necessitating its use.34
SSVD: There is a paucity of data regarding the primary prevention of SSVD due to an incomplete understanding of its pathogenesis. The most important intervention for primary prophylaxis of SVD is adequately controlled BP.13
SODE: Due to the heterogeneity of the etiologies of SODE, there are no general recommendations for primary prophylaxis of this type of vascular insult.6
Pharmacologic Therapy for Secondary Prevention of Stroke
In 2021, the AHA/ASA issued a guideline on the prevention of stroke in patients with stroke and TIA. The guideline provides recommendations for secondary prophylaxis (including on the use of antithrombotic medications) based on the etiology of the initial cerebral insult.33
CES: Factors to determine when starting DOAC therapy following an ischemic stroke include the risk of hemorrhagic transformation (in low-risk patients anticoagulation should be started between 2 and 14 days, and in high-risk patients, anticoagulation should be initiated after 2 weeks), the size of the infarct, and other patient factors, such as BP control.33
The 2021 AHA/ASA guideline on secondary stroke prevention recommends the following33:
• The use of DOACs or warfarin in patients with nonvalvular AFib and stroke or TIA to prevent recurrence.
• If moderate-to-severe mitral stenosis or a MHV is not present, DOACs are recommended over warfarin; they are also recommended if a therapeutic INR cannot be maintained in patients on warfarin. These recommendations also apply to patients with atrial flutter and stroke or TIA.
• Initiation of oral anticoagulation may be delayed beyond 2 weeks in patients at a high risk of hemorrhagic conversion.
• Anticoagulation should be initiated immediately after the event in patients with a TIA and nonvalvular AFib.
• If there is low risk of hemorrhagic conversion, anticoagulation can be initiated 2 to 14 days following the cerebral insult.
• In patients with AFib and stroke or TIA and who have end-stage renal disease or are on dialysis, warfarin or dose-adjusted apixaban is preferred.
The 2021 AHA/ASA guideline also provides recommendations on secondary stroke prevention in patients with valvular disease.33
The management for other risk factors for recurrent stroke vary from anticoagulation in heart failure in the presence of AFib to cardiac surgery in endocarditis.6
Cryptogenic Stroke: It is difficult to provide recommendations for secondary prophylaxis of cryptogenic stroke since there is wide range of possible underlying causes and the best approach to each may differ. In general, strategies include a combination of antiplatelet therapy, BP management, and lipid-lowering agents.11
Trials examining the role of DOACs for ESUS have been negative. Several trials have been halted early because of increased bleeding or futility.35-38 Anticoagulation is not recommended for secondary prevention of ischemic stroke in unselected patients with ESUS.6,11
SLAA: The same methodological issues discussed above in primary-prevention studies for SLAA also affect secondary-prevention trials. The benefit of DAPT appears to be greatest during the first 90 days following stroke, and after that the risk of bleeding outweighs benefit. Some of the treatment options that have been studied include ticagrelor monotherapy, low-dose prasugrel, clopidogrel monotherapy, and warfarin monotherapy, and triple therapy with aspirin, clopidogrel, and dipyridamole have demonstrated either a lack of benefit and/or an increased risk of bleeding.6
The 2021 AHA/ASA guideline on secondary stroke prevention distinguishes between intracranial LAA and extracranial LAA (i.e., carotid stenosis). Aspirin 325 mg/day is preferred over warfarin for intracranial LAA in patients with stroke or TIA caused by 50% to 99% stenosis of a major intracranial artery. If stroke or TIA occurred within the past 30 days and was due to severe stenosis (70%-90%) of a major intracranial artery, clopidogrel 75 mg/day can be added to aspirin therapy for up to 90 days. For patients who have a minor stroke or high-risk TIA within the past 24 hours and concomitant ipsilateral >30% stenosis of a major intracranial artery is present, ticagrelor 90 mg twice a day may be added to aspirin therapy for a maximum of 30 days. Cilostazol 200 mg/day may be added to aspirin or clopidogrel for patients with 50% to 90% stenosis of a major intracranial artery who have had a stroke or TIA, but the use of clopidogrel, ticagrelor, or cilostazol monotherapy or the combination of aspirin and dipyridamole is not well established. In extracranial LAA, carotid endarterectomy is recommended in patients with 70% to 99% ipsilateral severe carotid artery stenosis with a perioperative morbidity and mortality of <6%. Antiplatelet therapy, lipid-lowering therapy, and treatment of hypertension are recommended in patients with carotid artery stenosis and a history of stroke or TIA.33
In 2022, the American Academy of Neurology practice advisory subcommittee published a report on treatments to reduce the risk of recurrent stroke or death in patients with symptomatic large artery intracranial atherosclerosis or symptomatic intracranial atherosclerotic arterial stenosis (sICAS). The report issued three recommendations regarding antithrombotic therapy12:
• Aspirin 325 mg/day is recommended over warfarin for long-term prevention of stroke and death in patients with sICAS.
• Clopidogrel 75 mg/day added to aspirin for up to 90 days is recommended to further reduce stroke risk in severe (70%-99%) sICAS patients with a low risk of hemorrhagic transformation following ischemic stroke.
• Cilostazol 200 mg/day can be an alternative to clopidogrel and can be added to aspirin for up to 90 days to further reduce stroke risk in sICAS in those with low risk of hemorrhagic transformation or in Asian patients.
SSVD: There is significant evidence supporting the use of antiplatelet therapy, especially aspirin, in the prevention of SSVD.6 Aspirin was found to reduce the risk of recurrent stroke by 30% in patients who sustained an acute subcortical infarct.13 Prasugrel 3.75 mg/day was found to have similar efficacy and safety as clopidogrel 75 mg/day in various stroke types, including small artery occlusion.39 In a study in which <1/3 of the study population had SVD, ticagrelor (180 mg loading dose on Day 1 followed by 90 mg twice daily for Days 2 through 90) demonstrated similar efficacy and safety to aspirin (300 mg on Day 1 followed by 100 mg daily for Days 2 through 90).40
Results of studies exploring the use of dipyridamole and aspirin have been mixed. One trial found that aspirin 25 mg twice daily was equally effective as dipyridamole 200 mg twice daily in a modified-release form and that their concomitant use produced additive beneficial effects in preventing stroke recurrence.41 Aspirin 30-325 mg/day with dipyridamole 200 mg twice daily within 6 months of a TIA or minor stroke of presumed arterial origin was associated with an absolute risk reduction in stroke of 1.0% per year.42 Similar rates of stroke were observed in patients receiving 25 mg of aspirin plus 200 mg of extended-release dipyridamole twice daily or 75 mg of clopidogrel daily.43
The role of DAPT is less defined due to methodological problems with study design. Among the concerns are that the delay in initiation of therapy could have affected outcome; various doses of DAPT were used; stroke etiology was not defined; and the generalizability of the findings may not be applicable to all populations. The use of antiplatelets and anticoagulants is generally not advised in patients with cerebral amyloid angiopathy.6,13,44
Cilostazol has both mild antiplatelet effects and beneficial effects on endothelial function.6 A systematic review and meta-analysis found that cilostazol was effective for long-term secondary stroke prevention without increasing major bleeding risk, but questions remain regarding its generalizability due to its study population being limited to persons of Asia-Pacific heritage.45 Compared with aspirin, clopidogrel, ticlopidine, aspirin plus dipyridamole, and aspirin plus clopidogrel, cilostazol was found to be the most effective drug for secondary prevention of patients with lacunar strokes.46 However, the 2021 AHA/ASA guideline on secondary stroke prevention states that the usefulness of cilostazol for secondary stroke prophylaxis is uncertain.33
SODE: Secondary prophylaxis for SODE depends on the underlying cause. Antiplatelet therapy is used in moyamoya disease, which is a rare, progressive, cerebrovascular disorder due to blocked arteries in the basal ganglia; hypercoagulable conditions; fibromuscular dysplasia, which refers to abnormal development or growth of cells in the walls of the arteries resulting in narrowing or bulging vessels; or carotid web, “a rare form of focal intimal fibromuscular dysplasia, which is protruded into the lumen and forms a membrane-like shelf in the posterior aspect of the internal carotid artery bulb.”6,47-49
Anticoagulation is recommended as secondary prophylaxis of SODE in patients with active cancer, antiphospholipid syndrome, and some congenital heart diseases. In patients who have sustained an acute extracranial dissection, secondary prophylaxis should consist of either antiplatelet therapy with aspirin or anticoagulation with warfarin for the first 3 months following the neurologic insult.6
Pharmacist’s Role in Antithrombotic Management
There is a paucity of information about the role of the pharmacist in the management of antithrombotics in primary or secondary ischemic stroke prevention.50-52
Pharmacists need to take a proactive role in the management of patients with ischemic stroke, as drug-related problems have been identified in both community-dwelling persons with ischemic stroke and in hospitalized patients with the neurologic condition.53,54
Conclusion
Ischemic stroke is a leading cause of morbidity and mortality in the U.S. It is estimated that 90% of new cases of stroke are preventable. Pharmacists can play a major role in reducing patients’ risk of stroke by optimizing antihypertensive and hyperlipidemic therapies; collaborating on the management of underlying CVD; encouraging a healthy diet, exercise, smoking cessation, alcohol-consumption reduction, and stress relief; assisting with achieving goal hemoglobin A1c in patients with diabetes; and individualizing antithrombotic therapy for primary and secondary stroke prevention. Antithrombotic therapy places patients at increased risk of bleeding. Pharmacists should ensure the safe and optimal use of these agents.
REFERENCES
1. CDC. Stroke facts. www.cdc.gov/nchs/pressroom/sosmap/stroke_mortality/stroke.htm. Accessed November 5, 2023.
2. CDC. QuickStats: Age-adjusted death rates for stroke, by region- National Vital Statistics System, United States, 2001-2021. MMWR Morb Mortal Wkly Rep. 2023;72:1099.
3. CDC. Stroke mortality by state, 2021. www.cdc.gov/nchs/pressroom/sosmap/stroke_mortality/stroke.htm. Accessed November 5, 2023.
4. CDC. About stroke. www.cdc.gov/stroke/about.htm. Accessed November 5, 2023.
5. Unnithan AKA, Das JM, Mehta P. Hemorrhagic stroke. [Updated 2023 May 8]. In: StatPearls [Internet]. Treasure Island, FL: StatPearls Publishing; 2023.
6. Greco A, Occhipinti G, Giacoppo D, et al. Antithrombotic therapy for primary and secondary prevention of ischemic stroke: JACC state-of-the-art review. J Am Coll Cardiol. 2023;82(15):1538-1557.
7. Diener HC, Hankey GJ. Primary and secondary prevention of ischemic stroke and cerebral hemorrhage: JACC Focus Seminar. J Am Coll Cardiol. 2020;75(15):1804-1818.
8. Adams HP Jr, Bendixen BH, Kappelle LJ, et al. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke. 1993;24(1):35-41.
|9. Pillai AA, Tadi P, Kanmanthareddy A. Cardioembolic stroke. [Updated 2023 Jul 3]. In: StatPearls [Internet]. Treasure Island, FL: StatPearls Publishing; 2023.
10. Yaghi S, Bernstein RA, Passman R, et al. Cryptogenic stroke: research and practice. Circ Res. 2017;120(3):527-540.
11. Schulz UG. Cryptogenic stroke–how to make sense of a non-diagnostic entity. Maturitas. 2019;122:44-50.
12. Turan TN, Zaidat OO, Gronseth GS, et al. Stroke prevention in symptomatic large artery intracranial atherosclerosis practice advisory: report of the AAN Guideline Subcommittee. Neurology. 2022;98(12):486-498.
13. Cannistraro RJ, Badi M, Eidelman BH, et al. CNS small vessel disease: a clinical review. Neurology. 2019;92(24):1146-1156.
14. Science Direct. Lipohyalinosis. www.sciencedirect.com/topics/medicine-and-dentistry/lipohyalinosis. Accessed November 5, 2023.
15. Kim H, Kim JT, Lee JS, et al. Stroke of other determined etiology: results from the Nationwide Multicenter Stroke Registry. Stroke. 2022;53(8):2597-2606.
16. Kamarova M, Baig S, Patel H, et al. Antiplatelet use in ischemic stroke. Ann Pharmacother. 2022;56(10):1159-1173.
17. Kuo L, Chan YH, Liao JN, et al. Stroke and bleeding risk assessment in atrial fibrillation: where are we now? Korean Circ J. 2021;51(8):668-680.
18. MD Calc. CHA2DS2-VASC Score. www.mdcalc.com/calc/801/cha2ds2-vasc-score-atrial-fibrillation-stroke-risk . Accessed November 5, 2023.
19. Camm AJ, Lip GY, De Caterina R, et al; ESC Committee for Practice Guidelines (CPG). 2012 focused update of the ESC Guidelines for the management of atrial fibrillation: an update of the 2010 ESC Guidelines for the management of atrial fibrillation. Developed with the special contribution of the European Heart Rhythm Association. Eur Heart J. 2012;33(21):2719-2747.
20. GARFIELD-AF Risk Calculator. https://af.garfieldregistry.org/garfield-af-risk-calculator. Accessed November 5, 2023.
21. Fox KAA, Lucas JE, Pieper KS, et al; GARFIELD-AF Investigators. Improved risk stratification of patients with atrial fibrillation: an integrated GARFIELD-AF tool for the prediction of mortality, stroke and bleed in patients with and without anticoagulation. BMJ Open. 2017;7(12):e017157.
22. Wang C, Yu Y, Zhu W, et al. Comparing the ORBIT and HAS-BLED bleeding risk scores in anticoagulated atrial fibrillation patients: a systematic review and meta-analysis. Oncotarget. 2017;8(65):109703-109711.
23. Mitchell A, Elmasry Y, van Poelgeest E, et al. Anticoagulant use in older persons at risk for falls: therapeutic dilemmas-a clinical review. Eur Geriatr Med. 2023;14(4):683-696.
24. Pisters R, Lane DA, Nieuwlaat R, et al. A novel user-friendly score (HAS-BLED) to assess 1-year risk of major bleeding in patients with atrial fibrillation: the Euro Heart Survey. Chest. 2010;138(5):1093-1100.
25. O'Brien EC, Simon DN, Thomas LE, et al. The ORBIT bleeding score: a simple bedside score to assess bleeding risk in atrial fibrillation. Eur Heart J. 2015;36(46):3258-3264.
26. United States Preventive Services Task Force. Aspirin use to prevent cardiovascular disease: preventive medicine. April 26, 2022. www.uspreventiveservicestaskforce.org/uspstf/recommendation/aspirin-to-prevent-cardiovascular-disease-preventive-medication. Accessed November 5, 2023.
27. Eliquis (apixaban tablet) prescribing information. Princeton, NJ: Bristol-Myers Squibb, and New York, NY: Pfizer Laboratories, September 2021.
28. Savaysa (edoxaban tosylate) prescribing information. Basking Ridge, NJ: Daiichi-Sankyo; October 2023.
|29. Xarelto (rivaroxaban) prescribing information. Titusville, NJ: Janssen Pharmaceuticals, Inc; November 2023.
30. Pradaxa (dabigatran etexilate mesylate) prescribing information. Ridgefield, CT: Boehringer Ingelheim Pharmaceuticals, Inc. November 2023.
31. Joglar JA, Chung MK, Armbruster AL, et al. 2023 ACC/AHA/ACCP/HRS Guideline for the Diagnosis and Management of Atrial Fibrillation: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2023.
32. 2023 American Geriatrics Society Beers Criteria Update Expert Panel. American Geriatrics Society 2023 updated AGS Beers Criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2023;71(7):2052-2081.
33. Kleindorfer DO, Towfighi A, Chaturvedi S, et al. 2021 Guideline for the Prevention of Stroke in Patients With Stroke and Transient Ischemic Attack: a guideline from the American Heart Association/American Stroke Association. Stroke. 2021;52(7):e364-e467. Erratum in: Stroke. 2021;52(7):e483-e484.
34. Cole JW. Large artery atherosclerotic occlusive disease. Continuum (Minneapolis, MN). 2017;23(1):133-157.
|35. Hart RG, Sharma M, Mundl H, et al. Rivaroxaban for stroke prevention after embolic stroke of undetermined source. N Engl J Med. 2018;378:2191-2201.
36. Diener HC, Sacco RL, Easton JD, et al. Dabigatran for prevention of stroke after embolic stroke of undetermined source. N Engl J Med. 2019;380:1906-1917.
37. Poli S, Meissner C, Baezner HJ, et al. ATTICUS investigators, Apixaban for treatment of embolic stroke of undetermined source (ATTICUS) randomized trial–update of patient characteristics and study timeline after interim analysis. Eur Heart J. 2021;42(Suppl 1):ehab724.2070.
38. Clinicaltrials.gov. AtRial Cardiopathy and Antithrombotic Drugs In Prevention After Cryptogenic Stroke (ARCADIA), NCT03192215. https://clinicaltrials.gov/study/NCT03192215?tab=table. Accessed November 5, 2023.
39. Kitazono T, Toyoda K, Kitagawa K, et al; PRASTRO-I Study Group. Efficacy and safety of prasugrel by stroke subtype: a sub-analysis of the PRASTRO-I randomized controlled trial. J Atheroscler Thromb. 2021;28(2):169-180.
40. Johnston SC, Amarenco P, Albers GW, et al. SOCRATES Steering Committee and Investigators. Ticagrelor versus aspirin in acute stroke or transient ischemic attack. N Engl J Med. 2016;375(1):35-43.
41. Diener HC, Cunha L, Forbes C, et al. European Stroke Prevention Study. 2. Dipyridamole and acetylsalicylic acid in the secondary prevention of stroke. J Neurol Sci. 1996;143(1-2):1-13.
42. Halkes PH, van Gijn J, Kappelle LJ, et al. ESPRIT Study Group. Aspirin plus dipyridamole versus aspirin alone after cerebral ischaemia of arterial origin (ESPRIT): randomised controlled trial. Lancet. 2006;367(9523):1665-1673. Erratum in: Lancet. 2007;369(9558):274.
43. Sacco RL, Diener HC, Yusuf S, et al. PRoFESS Study Group. Aspirin and extended-release dipyridamole versus clopidogrel for recurrent stroke. N Engl J Med. 2008;359(12):1238-1251.
44. Hawkes MA, Braksick SA, Zhang W, et al. Can we stop the stuttering in stroke? Interventions in 40 patients with acute lacunes. J Neurol Sci. 2019;401:1-4.
45. McHutchison C, Blair GW, Appleton JP, et al. Cilostazol for secondary prevention of stroke and cognitive decline: systematic review and meta-analysis. Stroke. 2020;51(8):2374-2385.
46. Hou X, Cen K, Cui Y, et al. Antiplatelet therapy for secondary prevention of lacunar stroke: a systematic review and network meta-analysis. Eur J Clin Pharmacol. 2023;79(1):63-70.
47. National Institute of Neurological Disorders and Stroke. Moyamoya disease. www.ninds.nih.gov/health-information/disorders/moyamoya-disease. Accessed November 5, 2023.
48. National Institute of Neurological Disorders and Stroke. Fibromuscular dysplasia. www.ninds.nih.gov/health-information/disorders/fibromuscular-dysplasia. Accessed November 5, 2023.
49. Park HK, Hong KS. Carotid web: under-recognized etiology for ischemic stroke. J Neurosonol Neuroimag. 2018;10(2):100-105.
50. Bacchini M, Bonometti S, Del Zotti F, et al. Opportunistic screening for atrial fibrillation in the pharmacies: a population-based cross-sectional study. High Blood Press Cardiovasc Prev. 2019;26(4):339-344.
51. Chapman SA, St Hill CA, Little MM, et al. Adherence to treatment guidelines: the association between stroke risk stratified comparing CHADS2 and CHA2DS2-VASc score levels and warfarin prescription for adult patients with atrial fibrillation. BMC Health Serv Res. 2017;17(1):127.
52. Hohmann C, Neumann-Haefelin T, Klotz JM, et al. Adherence to hospital discharge medication in patients with ischemic stroke: a prospective, interventional 2-phase study. Stroke. 2013;44(2):522-524.
53. Tian L, Wu J, Qi Z, et al. Drug-related problems among community-dwelling elderly with ischemic stroke in China. Adv Clin Exp Med. 2023;32(4):423-432.
54. Chen Q, Jin Z, Zhang P, et al. Characteristics of drug-related problems among hospitalized ischemic stroke patients in China. Int J Clin Pharm. 2020;42(4):1237-1241.
The content contained in this article is for informational purposes only. The content is not intended to be a substitute for professional advice. Reliance on any information provided in this article is solely at your own risk.
To comment on this article, contact rdavidson@uspharmacist.com.






