ABSTRACT: Colchicine is a widely used medication that holds FDA approval for multiple indications. Colchicine 0.5 mg, with the branded name Lodoco, recently gained approval for an additional indication in June 2023 to reduce the risk of myocardial infarction, stroke, coronary revascularization, and cardiovascular death in adult patients with established atherosclerotic cardiovascular disease (ASCVD) or with multiple risk factors for cardiovascular disease. Colchicine was studied in various trials, including the COLCOT, LoDoCo, and LoDoCo2 trials, which evaluated the anti-inflammatory effects of colchicine on reducing cardiovascular events in patients with established ASCVD or patients at risk of developing ASCVD.
Atherosclerosis is a common inflammatory disease caused by the buildup of cholesterol, fat deposits, cellular waste products, calcium, and fibrin in the arteries.1 Many of the current therapies to reduce the risk of atherosclerotic cardiovascular disease (ASCVD) are based on mechanisms to lower cholesterol levels to reduce the buildup of cholesterol in the arteries. However, inflammation also has a critical role in ASCVD that is independent of cholesterol levels. The inflamed endothelium in the arteries attracts the migration, adhesion, and activation of leukocytes, the majority being neutrophils. C-reactive protein (CRP) and interleukin (IL)-6 are inflammatory biomarkers and are associated with an increased risk of cardiovascular (CV) events.2 The role of inflammation contributing to ASCVD led to various studies that explored the possibility that anti-inflammatory therapy may improve CV outcomes.
Colchicine was first approved in 1961 as an anti-inflammatory medication for the treatment of pain associated with gout.3 In 2009, a new formulation under the brand name Colcrys was approved by the FDA for the prevention of gout flares, as well as treatment of familial Mediterranean fever (FMF), and is typically prescribed in dosages of 0.6 mg by mouth two to four times a day.3-5 In June 2023, the FDA approved a low dosage of colchicine of 0.5 mg daily by mouth, under the brand name Lodoco, for reducing CV events in patients who already have ASCVD or those at risk of developing it.6
Colchicine is an oral anti-inflammatory medication that inhibits β-tubulin polymerization into microtubules, thereby preventing the activation, degranulation, and migration of neutrophils. Colchicine may also interfere with the intracellular assembly of the inflammasome complex in neutrophils and monocytes that mediates activation of IL-1β. These anti-inflammatory effects have also demonstrated that colchicine reduces high-sensitivity C-reactive protein (hs-CRP).2
The 2023 American Heart Association Guideline for the Management of Patients with Chronic Coronary Disease provides guidance for colchicine.7 It is recommended that in patients with chronic coronary disease (CCD), the addition of colchicine may be considered to reduce recurrent ASCVD events. The class of recommendation is class 2b (weak), indicating that colchicine may/might be considered reasonable, and the usefulness/effectiveness is unknown/unclear/uncertain or not well established. The level of evidence is B-R, indicating that there is moderate-quality evidence from one or more randomized, controlled trials or meta-analyses of moderate-quality randomized, controlled trials.7
Patients with stable coronary artery disease are at continued risk for major ASCVD events despite effective secondary prevention strategies, such as lifestyle changes and risk-factor reduction.7 It is recommended that colchicine be reserved for patients who remained at very high risk of ASCVD events despite maximum-tolerated, guideline-directed medication therapy (GDMT) for CCD. GDMT includes a β-hydroxy β-methylglutaryl coenzyme A (HMG-CoA) reductase inhibitor (statin), antiplatelet agent, beta-blocker, and a renin-angiotensin system (RAS) inhibitor. It is important to be aware that the recommended dosage of Lodoco for treatment of CCD is 0.5 mg daily based on the dose tested in clinical trials and is expected to be available as a brand-name medication only.7
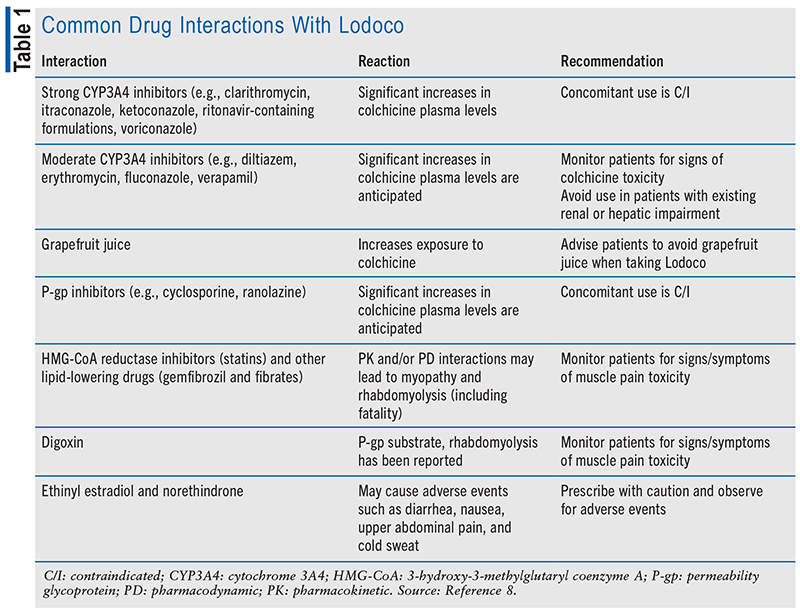
Lodoco is approved as a 0.5-mg tablet to be taken by mouth once a day with or without food.8 Overall, colchicine is well tolerated, with the two most common adverse reactions being gastrointestinal (GI) upset, including nausea, vomiting, and diarrhea (23%), and myalgia (21%).9 Patients should not use colchicine if they concomitantly take strong cytochrome 3A4 (CYP3A4) inhibitors or permeability glycoprotein (P-gp) inhibitors (clarithromycin, ketoconazole, or medications containing ritonavir) due to life-threatening and fatal colchicine toxicity. Patients with renal failure (creatinine clearance <15 mL/min), severe hepatic failure, or preexisting blood dyscrasias and patients who have a hypersensitivity to colchicine or any inactive ingredient of colchicine should avoid taking the medication.8
In addition, colchicine has many warnings and precautions listed.8 Colchicine can cause myelosuppression, leukopenia, granulocytopenia, thrombocytopenia, pancytopenia, and aplastic anemia, as well as neuromuscular toxicity and rhabdomyolysis. The concomitant use of colchicine with drugs that decrease the metabolism of colchicine, such as CYP3A4 inhibitors of P-gp inhibitors (e.g., clarithromycin, ketoconazole, or medications containing ritonavir), increases the risk of colchicine toxicity.8 Furthermore, the concomitant use of colchicine and HMG-CoA reductase inhibitors, gemfibrozil, and fenofibrate or cyclosporine may have the potential for development of myopathy. The presence of hepatic or renal impairment also increases the risk. GI symptoms are often the first sign of colchicine toxicity and should prompt evaluation. Males of reproductive potential should be advised that infertility from colchicine is rare and may be reversible.8
TABLE 1 outlines common drug interactions with Lodoco, the reaction, and recommendation.
RATIONALE
The CANTOS Trial
The Canakinumab Antiinflammatory Thrombosis Outcome Study (CANTOS) was a randomized, double-blind, placebo-controlled trial that investigated the use of canakinumab in high-risk patients with established ASCVD who have already survived a myocardial infarction (MI). The primary endpoint of the study was MI, stroke, or CV death.10 Canakinumab is an anti-IL-1β monoclonal antibody for SC injection that currently holds FDA approval for juvenile idiopathic arthritis and Still’s disease.11 The trial evaluated if the mechanism of reducing inflammation without reducing lipid levels may reduce the risk of cardiovascular disease (CVD). Patients were included if they were aged ≥18 years, had hs-CRP ≥2 mg/dL, and had an MI in the past 30 days. Patients were randomized to canakinumab 50 mg, 150 mg, or 300 mg versus placebo. Canakinumab 50 mg and 150 mg and placebo were administered SC once every 3 months; the 300-mg dosage was administered every 2 weeks for two doses, then once every 3 months.10
Overall, the CANTOS trial concluded that canakinumab 150 mg SC every 3 weeks led to a reduction in major adverse CV events compared with placebo, independent of lipid-level lowering.10 The primary endpoint of the incidence rate of an MI, stroke, or CV death at follow-up of 3.7 years was 4.50 events per 100-person years in the placebo group; 4.11 events per 100-person years in the canakinumab 50-mg group (hazard ratio [HR] 0.93; 95% CI, 0.80-1.07; P = .30); 3.89 events per 100 person-years in the 150-mg group (HR 0.85; 95% CI, 0.74-0.98; P = .02075 with a threshold P value of .02115); and 3.90 events per 100 person-years in the 300-mg group (HR 0.86; 95% CI, 0.75-0.99; P = .031, with a threshold P value of .01058).10 Despite these results, canakinumab did not receive FDA approval for CVD because canakinumab was associated with a higher incidence of fatal infection compared with placebo (incidence rate, 0.31 vs. 0.18 events per 100 person-years; P = .02).10-12 However, the results of this trial provided strong evidence of anti-inflammatory outcomes to treat ASCVD and led to the possibility of colchicine being studied for similar outcomes.
The COLCOT Trial
The Efficacy and Safety of Low-dose Colchicine after Myocardial Infarction (COLCOT) study was a randomized, double-blind trial that investigated 0.5 mg colchicine daily in patients within 30 days after an MI.13 A total of 4,745 patients were randomized, with 2,366 in the colchicine group and 2,379 patients in the placebo group. The study was conducted in 167 centers in 12 countries from December 2015 to August 2018 with a median 22.3-month follow-up. Patients were eligible if they were aged ≥18 years, had an MI within 30 days prior to enrollment, completed any planned percutaneous revascularization procedures, and were treated according to the national guidelines (antiplatelet therapy, statin therapy, RAS inhibitor, and beta-blocker). The mean age of patients was 60.6 years, 19.2% were women, and 72.5% were white. The patients were enrolled after a mean of 13.5 days from an MI, and 93.0% of patients underwent percutaneous coronary intervention for their MI. A total of 98.8% of patients were taking aspirin, 97.9% an antiplatelet agent other than aspirin, and 99.0% a statin.13
The primary endpoint of death from CV causes, resuscitated cardiac arrest, MI, stroke, or urgent hospitalization for angina leading to revascularization occurred in 131 (5.5%) patients in the colchicine group and 170 (7.1%) in the placebo group (HR 0.77; 95% CI, 0.61-0.96; P = .02). Overall, in patients with recent MI, colchicine 0.5 mg daily led to a significantly lower risk of ischemic CV events than placebo. The positive anti-inflammatory properties of the low-dose colchicine reported in this trial led to further studies, including LoDoCo and LoDoCo2.13
The LoDoCo and LoDoCo2 Trials
The Colchicine in Patients with Chronic Coronary Disease (LoDoCo) study was an open-label trial enrolling 532 patients—270 randomized to colchicine 0.5 mg and 250 to placebo. The LoDoCo trial found that the risk of acute CV events was lower in patients who received 0.5 mg of colchicine daily compared with placebo.14 The primary endpoint of the LoDoCo trial was the composite incidence of acute coronary syndrome (ACS), out-of-hospital cardiac arrest, or noncardioembolic ischemic stroke and occurred in 15 out of 282 patients (5.2%) who received colchicine and 40 out of 250 patients (16%) in the placebo group (HR 0.33; 95% CI, 0.18-0.59; P <.001). Overall, the LoDoCo trial concluded that colchicine 0.5 mg per day, in addition to statins and other standard secondary prevention therapies, is effective for the prevention of CV events in patients with stable coronary disease. The results of the LoDoCo trial led to the LoDoCo2 trial.14-16
LoDoCo2 was a multicenter, randomized, double-blind, placebo-controlled trial comparing colchicine 0.5 mg to placebo in adults with chronic coronary syndrome.15 A total of 5,522 patients were included in the LoDoCo2 trial, with 2,762 in the colchicine group and 2,760 in the placebo group, conducted at 33 centers in Australia and the Netherlands. The trial took place from August 2014 to December 2018, followed by 28.6 months for follow-up.15 The study included patients aged 35 to 82 years with coronary disease evidenced by coronary angiography, CT angiography, and coronary artery calcium (CAC) scan with a CAC score ≥400 Agatston units who were clinically stable for at least 6 months. Patients were excluded if they were pregnant/likely to be pregnant or breastfeeding, had renal impairment evidenced by a serum creatinine >150 mg/dL or estimated glomerular filtration rate <50 mL/min/1.73 m2, New York Heart Association functional classification ≥3, moderate or severe heart valve disease likely to require an intervention, dependency or frailty or a life expectancy <5 years, peripheral neuritis, myositis, marked myosensitivity to statins, had a requirement for long-term colchicine therapy, or enrollment in another trial.15
The trial had a run-in period in which patients received open-label colchicine 0.5 mg daily for 1 month. If they tolerated colchicine without unacceptable side effects, they were randomized to either colchicine 0.5 mg daily or placebo. The mean age of patients was 66 ± 8.6 years, 15.3% were female, 11.7% were current smokers and 18.2% had diabetes; 84.4% had a history of ACS, and in 68.2% of the patients, the ACS event occurred more than 24 months before randomization. A majority of patients (99.7%) were taking an antiplatelet agent or anticoagulation, 96.6% with a lipid-lowering agent, 62.1% a beta-blocker, and 71.7% a RAS inhibitor. The primary endpoint of CV mortality, spontaneous MI, ischemic stroke, or ischemia-driven coronary revascularization occurred in 6.8% of the colchicine group versus 9.6% of the placebo group (HR 0.69; 95% CI, 0.57-0.83; P <.001; number needed to treat = 36).15
Overall, the LoDoCo2 trial reported that among patients with CCD, colchicine 0.5 mg daily improved the CV outcomes. The results of the LoDoCo and LoDoCo2 trials are consistent with the results of the CANTOS and COLCOT trials that support the benefits of medications with anti-inflammatory properties to reduce the occurrence of CV events.10,13-16
THE ROLE OF THE PHARMACIST
Pharmacists should be aware of the distinct indication, prescribing information, dosing, timing, and duration of therapy for colchicine formulations. Lodoco is indicated for CCD at a dosage of 0.5 mg per day. Pharmacists can recommend this agent for patients who are at high risk for ASCVD despite GDMT. In addition, pharmacists should counsel patients on the adverse drug reactions and drug interactions.
CONCLUSION
In summary, colchicine has been used as an anti-inflammatory agent to treat mainly gout, FMF, and a new emerging indication for ASCVD risk reduction. In comparison with the brand Colcrys, Lodoco is recommended in patients with CCD to reduce recurrent ASCVD events. Pharmacists should be aware that the recommended dose of colchicine for treatment of CCD is 0.5 mg and is expected to be available as the brand name Lodoco only.
REFERENCES
1. American Heart Association. What is atherosclerosis? November 6, 2020. www.heart.org/en/health-topics/cholesterol/about-cholesterol/atherosclerosis. Accessed January 8, 2024.
2. Deftereos SG, Beerkens FJ, Shah B, et al. Colchicine in cardiovascular disease: in-depth review. Circulation. 2022;145(1):61-78.
3. LiverTox: Clinical and research information on drug-induced liver injury [Internet]. Bethesda, MD: National Institute of Diabetes and Digestive and Kidney Diseases; 2012-. Colchicine. [Updated 2017 Oct 26]. Available from: https://www.ncbi.nlm.nih.gov/books/NBK548068/
4. Colcrys (colchicine) package insert. Philadelphia, PA: AR Scientific, Inc.; 2009.
5. National Health Service. How and when to take colchicine. December 2, 2022. www.nhs.uk/medicines/colchicine/how-and-when-to-take-colchicine/. Accessed January 8, 2024.
6. Clinical Trials Arena. Lodoco (colchicine) for the treatment of cardiovascular disease, USA. July 24, 2023. www.clinicaltrialsarena.com/projects/lodoco-colchicine-cardiovascular-disease/?cf-view. Accessed November 21, 2023.
7. Virani SS, Newby K, Arnold SV, et al. 2023 AHA/ACC/ACCP/ASPC/NLA/PCNA Guideline for the Management of Patients With Chronic Coronary Disease: a report of the American Heart Association/American College of Cardiology Joint Committee on Clinical Practice Guidelines. J Am Coll Cardiol. 2023;82(9):833-955.
8. Lodoco (colchicine) package insert. Parsippany, NJ: Agepha Pharma USA, LLC; 2023.
9. Colchicine: drug information. In: Lexicomp online. Waltham, MA: UpToDate, Inc.; 2023. https://online.lexi.com. Accessed January 8, 2024.
10. Ridker PM, Everett BM, Thuren T, et al. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med. 2017;377(12):1119-1131.
11. Ilaris (canakinumab) package insert. East Hanover, NJ: Novartis Pharmaceuticals Corporation; 2009.
12. Lou N. FDA rejects canakinumab in CVD prevention. Medpage Today. 2018. www.medpagetoday.com/cardiology/prevention/75811. Accessed December 14, 2023.
13. Tardif JC, Kouz S, Waters D, et al. Efficacy and safety of low-dose colchicine after myocardial infarction. N Engl J Med. 2019;381(26):2497-2505.
14. Nidorf SM, Eikelboom JW, Budgeon CA, Thompson PL. Low-dose colchicine for secondary prevention of cardiovascular disease. J Am Coll Cardiol. 2013;61(4):404-410.
15. Nidorf SM, Fiolet ATL, Mosterd A, et al; LoDoCo2 Trial Investigators. Colchicine in patients with chronic coronary disease. N Engl J Med. 2020;383(19):1838-1847.
16. Nidorf SM, Fiolet ATL, Eikelboom JW, et al; LoDoCo2 Investigators. The effect of low-dose colchicine in patients with stable coronary artery disease: the LoDoCo2 trial rationale, design, and baseline characteristics. Am Heart J. 2019;218:46-56.
The content contained in this article is for informational purposes only. The content is not intended to be a substitute for professional advice. Reliance on any information provided in this article is solely at your own risk.
To comment on this article, contact rdavidson@uspharmacist.com.





