US Pharm. 2019;44(4):47-48.
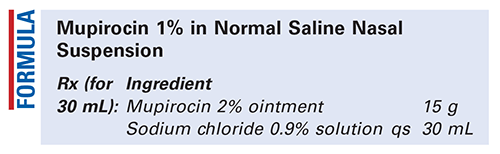
Method of Preparation: Calculate the quantity of each ingredient for the amount to be prepared. Accurately weigh or measure each ingredient. Accurately weigh the quantity of mupirocin 2% ointment. Add sufficient sodium chloride 0.9% solution slowly, with mixing, to final volume. Package and label.
Note: Nasal sprays and drops for topical application do not need to be sterile. They need to be sterile only if they are used for nasal inhalation.
Use: Mupirocin is an anti-infective agent used in the topical treatment of infections that are caused by Staphylococcus aureus, beta-hemolytic Streptococcus, and Streptococcus pyogenes.
Packaging: Package in either a nasal spray or dropper container.
Labeling: Keep out of reach of children. Shake well. For the nose. Discard after ____ [time period].
Stability: As a topically applied formulation, a beyond-use date of 30 days may be used for this preparation.1
Quality Control: Quality-control assessment can include weight/volume, pH, specific gravity, active drug assay, color, rheologic properties/pourability, physical observation, and physical stability (discoloration, foreign materials, gas formation, mold growth).2
Discussion: This preparation may be used as either a nasal spray or drops. It is important to clean the tip of the applicator nozzle or dropper following each administration.
Mupirocin (Bactroban, Centany, C26H44O9, MW 500.62) occurs as a white to off-white, crystalline solid that is freely soluble in dehydrated alcohol and very slightly soluble in water. This pseudomonic acid antibiotic produced by Pseudomonas fluorescens has a narrow spectrum of activity and is primarily employed against gram-positive bacteria. Mupirocin is used as a topical antibacterial agent in skin infections, especially impetigo, and in nasal carriage of methicillin-resistant S aureus.
Each gram of Bactroban 2% ointment contains 20 mg mupirocin in a water-miscible ointment base (polyethylene glycol ointment, NF) consisting of polyethylene glycol 400 and polyethylene glycol 3350.3 As discussed below, it is also available as mupirocin calcium in a cream and as nasal ointment containing paraffin and a mixture of glycerin esters (Softisan 649). However, the nasal ointment cannot be used for this nasal spray or drops because it does not mix well with the sodium chloride 0.9% solution.
Mupirocin calcium (Bactroban, MW 1075.34, C52H86CaO18.2H2O) occurs as a white to off-white, crystalline solid that is freely soluble in dehydrated alcohol and is very slightly soluble in water. Note that 2.15 mg of the calcium form is equivalent to 2 mg of mupirocin.3
Bactroban (mupirocin calcium) 2% nasal ointment contains the dihydrate crystalline hemicalcium salt of mupirocin. The nasal ointment is a white to off-white ointment that uses 2.15% w/w mupirocin calcium (equivalent to 2% mupirocin free acid) in a soft, white ointment base. The inactive ingredients are paraffin and a mixture of glycerin esters (Softisan 649).4
Bactroban (mupirocin calcium) 2% cream contains the dihydrate crystalline hemicalcium salt of mupirocin. Bactroban cream is a white cream that contains 2.15% w/w mupirocin calcium (equivalent to 2% mupirocin free acid) in an oil-in-water–based emulsion. The inactive ingredients are benzyl alcohol, cetomacrogol 1000, cetyl alcohol, mineral oil, phenoxyethanol, purified water, stearyl alcohol, and xanthan gum.5
Sodium chloride 0.9% solution contains not less than 95.0% and not more than 105.0% of the labeled amount of sodium chloride in purified water. (Note: Sodium Chloride 0.9% Injection or Sodium Chloride 0.9% for Irrigation may also be used in the formula.) Sodium chloride solutions are chemically and physically stable. Sodium chloride will decrease the solubility of some organic compounds; methylparaben is not as soluble in sodium chloride solutions as it is in water. Sodium chloride is soluble in water to the extent of 1 g in 2.8 mL water, and it is slightly soluble in alcohol (1 g in 250 mL of 95% ethanol).6
REFERENCES
1. U.S. Pharmacopeia/National Formulary [current revision]. Rockville, MD: U.S. Pharmacopeial Convention, Inc; March 2019.
2. Allen LV Jr. Standard operating procedure for performing physical quality assessment of oral and topical liquids. IJPC. 1999;3:146-147.
3. RxList. Bactroban ointment. www.rxlist.com/bactroban-ointment-drug.htm#indications. Accessed March 6, 2019.
4. GSKsource. Bactroban nasal. www.gsksource.com/bactroban_nasal. Accessed March 6, 2019.
5. GSKsource. Bactroban cream. https://gsksource.com/bactroban_cream. Accessed March 6, 2019.
6. Maximilien JS. Sodium chloride. In: Sheskey PJ, Cook WG, Cable CG, eds. Handbook of Pharmaceutical Excipients. 8th ed. London, England: Pharmaceutical Press; 2017:854-857.
To comment on this article, contact rdavidson@uspharmacist.com.





