US Pharm. 2022;47(1):24-33.
ABSTRACT: The oral chemotherapeutic agent (OCA) market has seen tremendous growth in recent years with the approval by the FDA of 15 novel OCAs from 2020 to June 2021. Most of these OCAs are dispensed through specialty pharmacies because of their high cost, complex dosing regimens, adverse event profiles, and need for close supervision. This article will describe the mechanisms of action, special considerations for the safe administration, and cost of recently approved OCAs. It will also describe the role of specialty pharmacists in promoting adherence and safe administration of OCAs to optimize patient outcomes.
According to the National Comprehensive Cancer Network, the goals of specialty pharmacy (SP) include “the optimization of pharmaceutical care outcomes through ensuring appropriate medication use, maximizing medication adherence, and optimizing economic outcomes through avoiding unwarranted drug expenditures.”1 Perhaps, there is no patient population that can benefit more from the achievement of such goals than cancer patients receiving oral chemotherapeutic agents (OCAs).
The use of OCAs offers numerous benefits, including convenience, enhanced patient autonomy and privacy, less disruption in a patient’s daily schedule, lower healthcare resources, elimination of the need for port placement that may be required for IV chemotherapeutic agents, and avoidance of transportation issues. There are disadvantages to the outpatient use of OCAs as well. The dispensing of potent OCAs in the SP setting poses unique challenges involving inadequate patient health literacy/patient education for the safe self-administration of these toxic medications, nonadherence that can lead to increased adverse effects due to unnecessary dose escalations, incomplete information about a patient’s drug regimen, including non-OCAs and pharmacologic supportive measures, lack of recognition of drug-drug or drug-food interactions, failure in identifying serious adverse events and how to manage them, and unfamiliarity with proper storage and disposal techniques. There may also be restricted formularies and supply chains that can delay treatment initiation, miscommunication between patients and/or healthcare providers about how to safely administer these medications, failure to communicate any schedule or dosage changes that may result from adverse effects or laboratory abnormalities, and financial toxicity.1-4 Specialty pharmacists can play a pivotal role in helping oncology patients navigate these obstacles in order to achieve a cytogenic response, remission, or cure.
Rapid Growth of the OCA Market and the Role of Specialty Pharmacies
By 2022, it is expected that $100 billion of U.S. healthcare spending will be for oncology medications, many of which are specialty drugs.5 Spending on specialty drugs in 2020 accounted for 49.6% of total prescription expenditures from all sectors.6 About 30% of all oncology drugs in the research pipeline are OCAs.7
Mechanisms of Recent FDA OCA Approvals
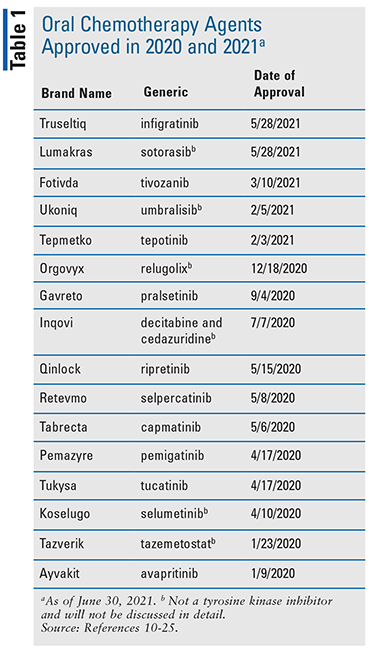
From 2020 through June 2021, the FDA approved 15 novel OCAs (TABLE 1).8,9 These agents often have multiple complex mechanisms of action. While a detailed discussion of the various mechanisms of action is beyond the scope of this article, a brief discussion will be provided.
Tyrosine Kinase Inhibitors
Most of the oral agents discussed in this article are tyrosine kinase inhibitors (TKIs). TKIs are a group of pharmacological agents that block the signal-transduction pathways of protein kinases by inhibiting phosphorylation of amino acids, thereby affecting cell growth, migration, differentiation, apoptosis, and tumor cell death.26,27
Additional mechanisms of action for the recently approved OCAs can be found in SIDEBAR 1.
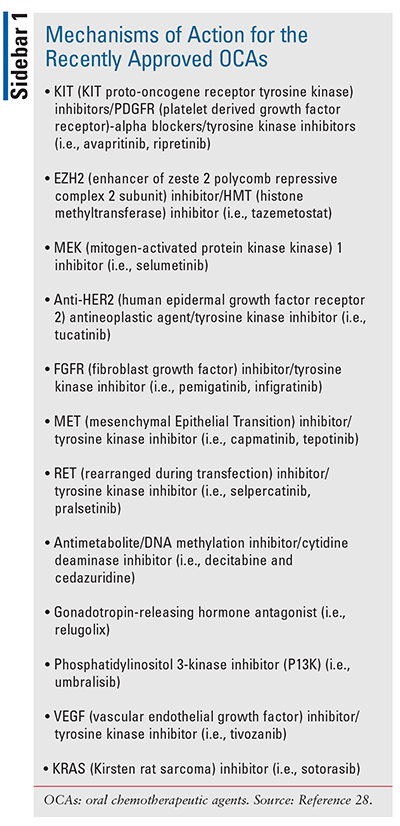
KIT Proto-Oncogene Receptor Tyrosine Kinase Platelet–Derived Growth Factor Receptor: The KIT gene is involved in the production of receptor tyrosine kinases. KIT protein signaling is involved in cell proliferation, survival, and migration. It is important in the development and function of germ cells, hematopoietic stem cells, mast cells, melanocytes, and cells in the gastrointestinal tract called interstitial cells of Cajal (ICCs). KIT gene mutations are involved in the development of piebaldism (i.e., a condition characterized by white patches of skin and hair due to lack of melanocytes), gastrointestinal stromal tumors (GISTs), systemic mastocytosis (i.e., a hematological disorder characterized by mast cells abnormally accumulating in tissue), as well as some cases of acute myeloid leukemia, sinonasal natural killer/T-cell lymphoma, and seminomas.29
The PDGFRA gene provides instruction to produce platelet-derived growth factor receptor alpha (PDGFRA), which is a receptor tyrosine kinase. Mutations in the PDGFRA gene are associated with the development of PDGFRA-associated chronic eosinophilic leukemia, GISTs, and inflammatory fibroid polyps.30
Avapritinib and ripretinib are two oral chemotherapeutic agents that are KIT/ PDGFR TKIs. These are the first two drugs marketed that are KIT inhibitors (TABLE 1).18,25,28
Human Epidermal Growth Factor Receptor 2: The human epidermal growth factor receptorn 2 (HER2) gene makes the HER2 protein, which is found on the surface of all breast cancer cells and is involved in normal cell growth. In HER2-positive breast cancer, the HER2 protein is overexpressed.31
Tucatinib is an anti-HER2 antineoplastic agent/TKI.22,32 It provides additional HER2 blockade and is used in combination with trastuzumab and capecitabine in patients with advanced unresectable or metastatic HER2-positive breast cancer, including patients with brain metastases, who have received prior anti-HER2 treatments.33 Tucatinib significantly improves progression-free survival at 1 year and overall survival at 2 years, as well as progression-free survival at 1 year in patients with brain metastases (TABLE 1).34
Fibroblast Growth Factor: The fibroblast growth factor (FGFR)3 gene is involved in the production of the protein, fibroblast growth factor receptor 3, which plays a role in the regulation of cell proliferation, determination of cell type, angiogenesis, wound healing, and embryo development. FGFR3 protein also regulates bone growth by limiting ossification. An isoform of this protein is found in epithelial skin cells in the epidermis.35
The FGFR pathway has been implicated in about 7% of cancers.36 Conditions associated with mutations in the FGFR3 gene include achondroplasia (i.e., a form of short-limbed dwarfism), Crouzon syndrome with acanthosis nigricans (i.e., a condition involving premature joining of the bones of the skull [i.e., craniosynostosis], misshapen head, distinguishable facial features, and darkening of skin in body folds), epidermal nervus (i.e., abnormal skin growths composed of keratinocytes), hypochondroplasia (i.e., a form of short-limbed dwarfism), lacrimo-auriculo-dento-digital syndrome (i.e., a syndrome involving abnormal tear production, malformed eyes, hearing impairment, decreased saliva production, small teeth, and hand deformities), Muenke syndrome (i.e., a condition that involves craniosynostosis), SADDAN (also known as severe achondroplasia with developmental delay and acanthosis nigricans), thanatophoric dysplasia (i.e., a condition associated with extremely short limbs and a narrow chest), bladder cancer, multiple myeloma, CATSHL syndrome (camptodactyly, tall stature, hearing loss syndrome), and cervical cancer.35
Dermatologic adverse effects are a class effect of FGFR inhibitors. These adverse reactions include onycholysis (i.e., separation of the nail from the nail bed), palmar-plantar erythrodysesthesia syndrome (also known as foot-hand syndrome), stomatitis, alopecia, xerostomia, dysgeusia, mucositis, and calciphylaxis (i.e., calcium deposits in small blood vessels of the adipose and skin tissue). Guidelines were recently published on the prevention and management of these adverse skin effects.37
Resistance has also been reported with FGFR inhibitors and is due to several mechanisms, including FGFR binding-site mutations, gatekeeper-gene mutations, acquired kinase domains, and activation of alternative pathways, including epithelial growth factor receptor (EGFR), P13K, and MEK pathways.36
Erdafitinib is an oral FGFR inhibitor/TKI that was approved in 2019 for locally advanced or metastatic urothelial carcinoma with susceptible FGFR genetic alteration.28
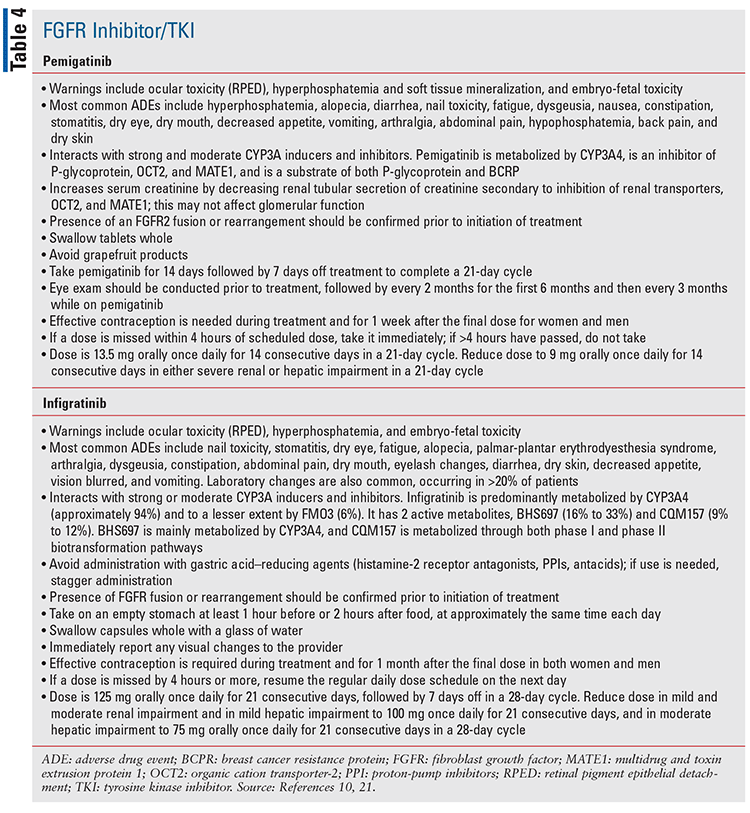
Infigratinib and pemigatinib are oral FGFR inhibitor/TKIs; both are indicated for unresectable, locally advanced, or metastatic cholangiocarcinoma with susceptible FGFR2 genetic alterations (TABLE 1).10,21,28
Mesenchymal-Epithelial Transition Factor: c-Mesenchymal-epithelial transition factor (MET) belongs to the MET family and is a type of receptor tyrosine kinase that is expressed on the surfaces of epithelial cells. It binds to ligand hepatocyte growth factor/scatter factor (HGF/SF), which is a member of the plasminogen-related growth factor family. HGF/c-Met regulates embryo development, tissue regeneration, wound healing, and the formation of nerve and muscle; however, abnormal activation of c-Met promotes tumor growth, including cancers of the liver, lung, colon, breast, pancreas, ovary, prostate, stomach, and the nervous system, such as glioblastoma. Elevated levels of c-Met expression are closely associated with poor prognosis in cancer patients.38
A recent review examined current and future treatment options for MET exon 14 skipping alterations in non-small cell lung cancer.39
Amivantamab is an injectable monoclonal antibody that acts as an MET inhibitor, but it is also an EGFR inhibitor. It is approved for locally advanced or metastatic non-small cell lung cancer with epidermal growth factor receptor exon 20 insertion mutation.28
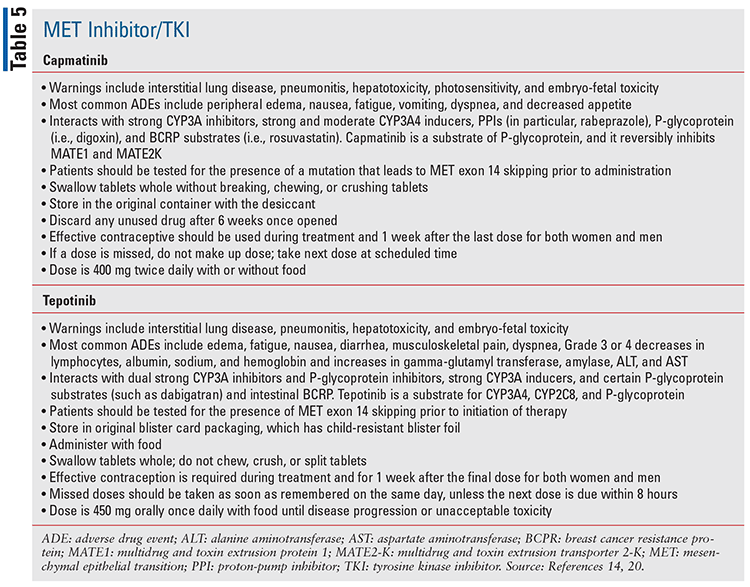
Capmatinib and tepotinib are both oral MET inhibitor/TKIs, which are approved for metastatic non-small cell lung cancer with MET exon 14 skipping mutation (TABLE 1).14,20,28
Rearranged During Transfection: The rearranged during transfection (RET) gene codes RET protein, which is involved in the development of nerve cells (including the enteric neurons and the autonomic nervous system), normal kidney development, and spermatogenesis. Mutations in the RET gene cause pheochromocytomas, Hirschsprung’s disease (i.e., a condition associated with severe constipation and intestinal obstruction), endocrine neoplasias, lung cancer, and thyroid cancers.40,41
Several recent reviews have been published on the role of RET-associated cancers.42-44
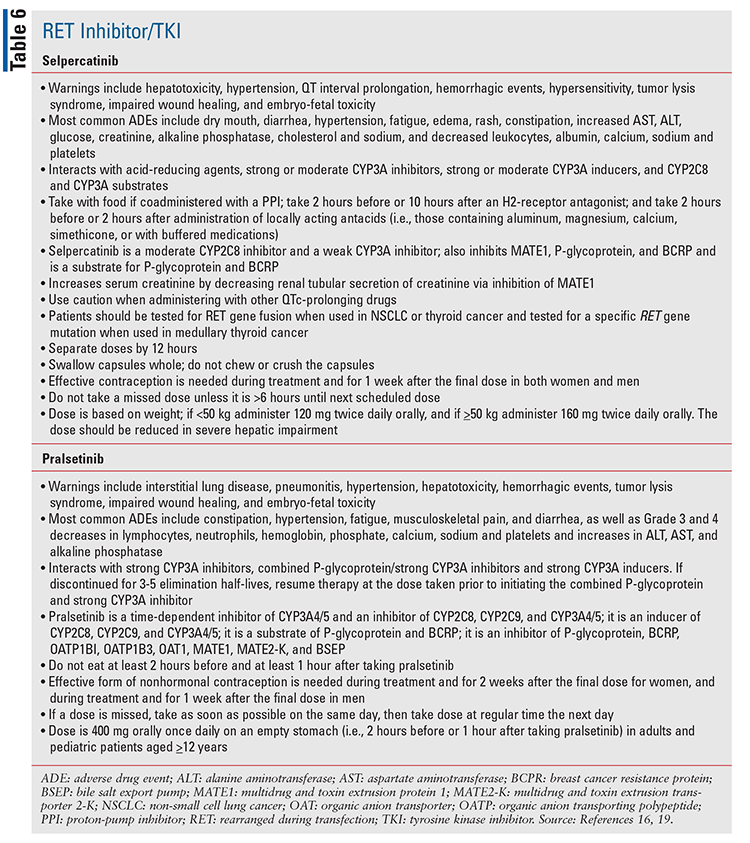
Selpercatinib and pralsetinib are two oral RET inhibitor/TKIs that are approved for metastatic RET fusion–positive non-small cell lung cancer, advanced or metastatic RET fusion–positive thyroid cancer, and advanced or metastatic RET–mutant medullary thyroid cancer (see TABLE 1).16,19,28
Vascular Endothelial Growth Factor: Vascular endothelial growth factor (VEGF) is active in angiogenesis, vasculogenesis, and endothelial cell growth. It induces endothelial cell proliferation, promotes cell migration, inhibits apoptosis, and induces permeabilization of blood vessels involved in tumor growth.45 Since many solid tumors overexpress VEGF, inhibiting this growth factor produces antiangiogenesis effects by disrupting the blood supply to the tumor and essentially starving it of nutrients and oxygen, thereby suppressing tumor growth.46 As a class, VEGF inhibitors cause generalized endothelial dysfunction, which predisposes to vasoconstriction, atherosclerosis, platelet activation, and thrombosis.47 A recent Bayesian network analysis found that in general, this class of agents is associated with severe cardiovascular incidents and hypertension.48
There are numerous VEGF inhibitors available, including monoclonal antibodies bevacizumab and ramucirumab, as well as axitinib, cabozantinib, lenvatinib, pazopanib, regorafenib, sorafenib, sunitinib, and vandetanib. These are approved for a variety of oncologic indications.28,49
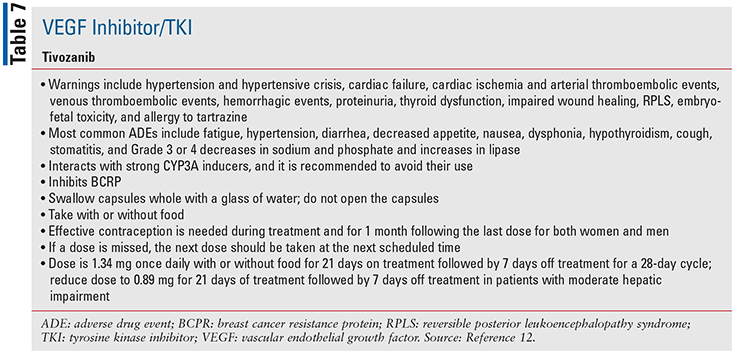
Tivozanib is an oral VEGF/TKI that is indicated for advanced, relapsed, or refractory renal cell carcinoma (TABLE 1).12,28
Special Considerations With Specific OCAs
Special considerations regarding specific OCAs that have recently been approved are discussed in TABLES 2 through 7.


Reining in Cost of OCAs: Role of Specialty Pharmacies
A study by the Community Oncology Alliance and Avalere Health examining access to OCAs found that patients were more likely to abandon their OCA treatment regimen as cost-sharing increased. This was especially common in those with lower incomes, as patients with annual incomes lower than $40,000 were 20% more likely to discontinue treatment than those whose incomes were higher than $75,000.50
OCAs are covered under Medicare Part D, and this has resulted in Medicare beneficiaries experiencing higher cost-sharing than commercially insured patients. This has contributed to a greater rate of OCA abandonment among those in the federal program compared with patients with commercial insurance (16% vs. 9%, respectively).50 Approximately 85% of Medicare Part D plans place OCAs in specialty tiers.50 This can result in an out-of-pocket expense for Medicare Part D beneficiaries of 25% to 33%, posing an undue financial hardship for patients struggling with a life-threatening illness.3 In 2019, it was found that the average out-of-pocket annual expense for an OCA for a Medicare Part D beneficiary was $10,470, which was an increase of $1,676 from 2010.51
An inverse relationship has been demonstrated between adherence to an OCA and cost with compliance decreasing as drug costs increase. A study using administrative claims data found that with cost sharing of greater than $500, oncology patients were four times more likely to abandon OCA treatment compared with cost sharing of less than $100.52
Split or partial fill of an OCA prescription may help reduce cost if the medication is changed, or if the drug is discontinued due to adverse events or treatment failure.53 However, a drawback is that this type of fill may result in a delay in treatment if the rest of the prescription order does not arrive in time for the subsequent dosing.
Specialty Pharmacist’s Role in the Management of Oral Chemotherapy
Specialty pharmacists are in an opportune position to assist patients in overcoming many of the barriers and challenges associated with the safe use of OCAs. These include nonadherence, financial burden, problems with drug-food interactions, and polypharmacy. Including specialty trained oncology pharmacists (e.g., board-certified oncology pharmacists) can help ensure best practices in OCA treatment management.54 Establishing collaborative medication management programs involving OCAs is another avenue to expand the role of the specialty pharmacist.55
There are numerous factors that can contribute to nonadherence, including poor health literacy, cognitive impairment, depression, asymptomatic disease, inadequate follow-up or discharge planning, adverse drug events, patient beliefs in the lack of benefit of treatment, lack of disease insight, complex OCA dosing schedules, lack of a provider-patient relationship/communication, inadequate social support, and cost; cost is a major risk factor for nonadherence.56,57 Helping defray the financial burden of OCAs is an important role of specialty pharmacists. Delays in drug approval may also occur because of cost.58 Untoward effects associated with nonadherence of OCAs include increased hospitalization, lengthier hospital stays, compromised disease outcomes, increased physician visits, and increased morbidity and mortality.56 Specialty pharmacists can help patients navigate obstacles associated with nonadherence with the use of manufacturer-provided patient assistance programs, copay cards, and foundation grants.
Overcompliance with OCAs, which has been found to have a prevalence of 20%, may be due to failure in drug discontinuation when the course of therapy is completed. This is more likely to occur when the dosing regimen is complex.59 This can be addressed through appropriate patient/caregiver education.
Food-drug interactions are a major concern with the use of OCAs. One study found that of the 58 OCAs examined, over 34% were to be taken on an empty stomach, whereas 16% were to be taken with food. This can be very confusing to patients who are on multiple medications and processing other information regarding their serious illness.60
Polypharmacy is also a concern with the use of OCAs. This is especially in older adults who may be on multiple chronic medications and who utilize different pharmacies for their nonspecialty medications.61 Over one-third of cancer patients may use complementary and alternative medicine, particularly herbal supplements, which may interact with OCAs.62
Patient education and tailored and supportive interventions have been shown to significantly improve cytogenic responses and survival times in patients with cancer by promoting adherence.63
Patients who are receiving complex OCA regimens that involve variable week on-off dosing schedules or who must combine different strengths to make the desired dose are especially vulnerable to making medication errors. Patient education is essential to avoid administration issues with OCAs, which can result in bone marrow suppression and compromised treatment efficacy or death.64
Failure to recognize the presence of adverse drug events has resulted in more than 20% of OCA-related adverse drug events not being reported to healthcare providers; this includes grade 3 adverse events. Through education and follow-up phone calls, specialty pharmacists can help identify these adverse events and promptly communicate them to patients’ providers.65
Conclusion
Specialty pharmacists can play a key role in the safe and effective use of OCAs by engaging in patient/caregiver education and assessing patient medication knowledge, proactively monitoring for adverse drug events and laboratory abnormalities that can impact OCA therapy, and recommending dose/regimen adjustments based on patient-specific factors (e.g., pharmacogenomics), as well as suggesting the use of supportive measures when applicable and facilitating the procurement of financial assistance through the submission of prior authorizations and collaborating with pharmaceutical assistance programs.
REFERENCES
1. Schwartz RN, Eng KJ, Frieze DA, et al. NCCN Task Force Report: specialty pharmacy. J Natl Comp Canc Netw. 2010;8(4):S1-S12.
2. Neuss MN, Polovich M, McNiff K, et al. 2013 updated American Society of Clinical Oncology/Oncology Nursing Society chemotherapy administration safety standards including standards for the safe administration and management of oral chemotherapy. [Erratum in: J Oncol Pract. 2013;9(5):265.] J Oncol Pract. 2013;9(2 Suppl):5s-13s.
3. Royce TJ, Schenkel C, Kirkwood K, et al. Impact of pharmacy benefit managers on oncology practices and patients. JCO Oncol Pract. 2020;16(5):276-284.
4. Wyatt H, Peter M, Zuckerman A, et al. Assessing the impact of limited distribution drug networks based on time to accessing oral oncolytic agents at an integrated specialty pharmacy. J Hematol Oncol Pharm. 2020;10(4):198-205.
5. Green AK, Ohn JA, Bach PB. Review of current policy strategies to reduce US cancer drug costs. J Clin Oncol. 2020;38(4):372-379.
6. Tichy EM, Hoffman JM, Suda KJ, et al. National trends in prescription drug expenditures and projections for 2021. Am J Health Syst Pharm. 2021;78(14):1294-1308.
7. Hematology/Oncology Pharmacy Association (HOPA). Oral chemotherapy issue brief. www.hoparx.org/images/hopa/advocacy/Issue-Briefs/HOPA_Oral_Chemotherapy_Issue_Brief.pdf. Accessed November 5, 2021.
8. FDA. Novel drug approvals for 2020. https://www.fda.gov/drugs/new-drugs-fda-cders-new-molecular-entities-and-new-therapeutic-biological-products/novel-drug-approvals-2020. Accessed November 5, 2021.
9. FDA. Novel drug approvals for 2021. https://www.fda.gov/drugs/new-drugs-fda-cders-new-molecular-entities-and-new-therapeutic-biological-products/novel-drug-approvals-2021. Accessed November 5, 2021.10. Truseltiq (infigratinib capsule) prescribing information. Brisbane, CA: QED Therapeutics, Inc.; May 2021.
11. Lumakras (sotorasib tablet, coated) prescribing information. Thousand Oaks, CA: Amgen Inc.; May 2021.
12. Fotivda (tivozanib capsule) prescribing information. Kansas City, MO: AVEO Pharmaceuticals, Inc.; March 2021.
13. Ukoniq (umbraslisib tablet, film coated) prescribing information. New York, NY: TG Therapeutics, Inc.; February 2021.
14. Tepmetko (tepotinib hydrochloride tablet) prescribing information. Rockland, MA: EMD Serono, Inc.; February 2021.
15. Orgovyx (relugolix tablet, film coated) prescribing information. Brisbane, CA: Myovant Sciences, Inc.; December 2020.
16. Gavreto (pralsetinib capsule) prescribing information. South San Francisco, CA: Genentech Inc.; April 2021.
17. Inqovi (cedazuridine and decitabine tablet, film coated) prescribing information. Princeton, NJ: Taiho Pharmaceutical Co., Ltd; July 2020.
18. Qinlock (ripretinib tablet) prescribing information. Waltham, MA: Deciphera Pharmaceuticals, Inc.; June 2021.
19. Retevmo (selpercatinib capsule) prescribing information. Indianapolis, IN: Eli Lilly and Company; January 2021.
20. Tabrecta (capmatinib table, film coated) prescribing information. East Hanover, NJ: Novartis Pharmaceuticals Corporation; May 2020.
21. Pemazyre (pemigatinib tablet) prescribing information. Wilmington, DE: Incyte Corporation; June 2021.
22. Tukysa (tucatinib tablet) prescribing information. Bothell, WA: Seagen Inc.; April 2020.
23. Koselugo (selumetinib capsule) prescribing information. Wilmington, DE: AstraZeneca Pharmaceuticals LP; May 2021.
24. Tazverik (tazemetostat tablet, film coated) prescribing information. Cambridge, MA: Epizme, Inc.; July 2020.
25. Ayvakit (avapritinib tablets, film coated) prescribing information. Cambridge, MA; Blueprint Medicines Corporation; June 2021.
26. Thomson RJ, Moshirfar M, Ronquillo Y. Tyrosine kinase inhibitors. [Updated 2021 May 4]. In: StatPearls [Internet]. Treasure Island, FL: StatPearls Publishing. https://www.ncbi.nlm.nih.gov/books/NBK563322/. Accessed November 5, 2021.
27. Mandal A. What is a kinase inhibitor? https://www.news-medical.net/life-sciences/What-is-a-Kinase-Inhibitor.aspx. Accessed November 5, 2021.
28. Wolter Kluwer. Lexicomp Mobile app.
29. National Institutes of Health. MedlinePlus. KIT gene. www.medlineplus.gov/genetics/gene/kit/. Accessed November 5, 2021.
30. National Institutes of Health. MedlinePlus. PDGFRA gene. www.medlineplus.gov/genetics/gene/pdgfra/. Accessed November 5, 2021.
31. National Institutes of Health. MedlinePlus. HER2 (breast cancer) testing. www.medlineplus.gov/lab-tests/her2-breast-cancer-testing/. Accessed November 5, 2021.
32. Ulrich L, Okines AFC. Treating advanced unresectable or metastatic HER2-positive breast cancer: a spotlight on tucatinib. Breast Cancer (Dove Med Press). 2021;13:361-381.
33. FDA. FDA approves tucatinib for patients with HER2-positive metastatic breast cancer. www.fda.gov/drugs/resources-information-approveddrugs/fda-approves-tucatinib-patients-her2-positive-metastatic-breast-cancer. Accessed November 5, 2021.
34. Murthy RK, Loi S, Okines A, et al. Tucatinib, trastuzumab, and capecitabine for HER2-positive metastatic breast cancer. [Erratum in: N Engl J Med. 2020;382(6):586]. N Engl J Med. 2020;382(7):597-609.
35. National Institutes of Health. MedlinePlus. FGFR3 gene. www.medlineplus.gov/genetics/gene/fgfr3/. Accessed November 5, 2021.
36. Kommalapati A, Tella SH, Borad M, et al. FGFR inhibitors in oncology: insight on the management of toxicities in clinical practice. Cancers (Basel). 2021;13(12):2968.
37. Lacouture ME, Sibaud V, Anadkat MJ, et al. Dermatologic adverse events associated with selective fibroblast growth factor receptor inhibitors: overview, prevention, and management guidelines. Oncologist. 2021;26(2):e316-e326.
38. Zhang Y, Xia M, Jin K, et al. Function of the c-Met receptor tyrosine kinase in carcinogenesis and associated therapeutic opportunities. Mol Cancer. 2018;17(1):45.
39. Hong L, Zhang J, Heymach JV, Le X. Current and future treatment options for MET exon 14 skipping alterations in non-small cell lung cancer. Ther Adv Med Oncol. 2021;13:1758835921992976.
40. National Institutes of Health. MedlinePlus. RET gene. www.medlineplus.gov/genetics/gene/ret/. Accessed November 5, 2021.
41. Markham A. Selpercatinib: first approval. Drugs. 2020;80(11):1119-1124.
42. Subbiah V, Yang D, Velcheti V, et al. State-of-the-art strategies for targeting RET-dependent cancers. J Clin Oncol. 2020;38(11):1209-1221.
43. Drusbosky LM, Rodriguez E, Dawar R, et al. Therapeutic strategies in RET gene rearranged non-small cell lung cancer. J Hematol Oncol. 2021;14(1):50.
44. Stinchcombe TE. Current management of RET rearranged non-small cell lung cancer. Ther Adv Med Oncol. 2020;12:1758835920928634.
45. GeneCards: The Human Gene Database. VEGFA gene. www.genecards.org/cgi-bin/carddisp.pl?gene=VEGFA&keywords=VEGF,inhibitors. Accessed November 5, 2021
46. Zirlik K, Duyster J. Anti-angiogenics: current situation and future perspectives. Oncol Res Treat. 2018;41(4):166-171.
47. Touyz RM, Herrmann SMS, Herrmann J. Vascular toxicities with VEGF inhibitor therapies–focus on hypertension and arterial thrombotic events. J Am Soc Hypertens. 2018;12(6):409-425.
48. Hou W, Ding M, Li X, et al. Comparative evaluation of cardiovascular risks among nine FDA-approved VEGFR-TKIs in patients with solid tumors: a Bayesian network analysis of randomized controlled trials. J Cancer Res Clin Oncol. 2021;147:2407-2420.
49. Qin S, Li A, Yi M, et al. Recent advances on antiangiogenesis receptor tyrosine kinase inhibitors in cancer therapy. J Hematol Oncol. 2019;12(1):27.
50. Community Oncology Alliance. Fact Sheet: Patient access to oral oncolytics. https://communityoncology.org/pdfs/fact-sheet-oral-oncolytics.pdf. Accessed November 5, 2021.
51. Dusetzina SB, Huskamp HA, Keating NL. Specialty drug pricing and out-of-pocket spending on orally administered anticancer drugs in Medicare Part D, 2010 to 2019. JAMA. 2019;321(20):2025-2028.
52. Streeter SB, Schwartzberg L, Husain N, Johnsrud M. Patient and plan characteristics affecting abandonment of oral oncolytic prescriptions. Am J Manag Care. 2011;17(suppl 5 developing):SP38-SP44.
53. Khandelwal N, Duncan I, Ahmed T, et al. Oral chemotherapy program improves adherence and reduces medication wastage and hospital admissions. J Natl Compr Canc Netw. 2012;10(5):618-625.
54. Holle LM, Bilse T, Alabelewe RM, et al. International Society of Oncology Pharmacy Practitioners (ISOPP) position statement: role of the oncology pharmacy team in cancer care. J Oncol Pharm Pract. 2021;27(4):785-801.
55. Passey DG, Healy R, Qualls J, et al. Pharmacist-led collaborative medication management programs for oral antineoplastic therapies: a systematic literature review. J Am Pharm Assoc (2003). 2021;61(3):e7-e18.
56. D’Amato S. Improving patient adherence with oral chemotherapy. Oncol Issues. 2008;23:4,42-45.
57. Thomas (Patel) SA, John T, Criner E, Nguyen TM. Challenges to oral chemotherapy adherence. US Pharm. 2019;44(6):HS-9-HS-12.
58. Doshi JA, Li P, Huo H, et al. Association of patient out-of-pocket costs with prescription abandonment and delay in fills of novel oral anticancer agents. J Clin Oncol. 2018;36(5):476-482.
59. Spoelstra SL, Given BA, Given CW, et al. Issues related to overadherence to oral chemotherapy or targeted agents. Clin J Oncol Nurs. 2013;17(6):604-609.
60. Segal EM, Flood MR, Mancini RS, et al. Oral chemotherapy food and drug interactions: a comprehensive review of the literature. J Oncol Pract. 2014;10(4):e255-e268.
61. Whitman A, Erdeljac P, Jones C, et al. Managing polypharmacy in old adults with cancer across different healthcare settings. Drug Healthc Patient Saf. 2021;13:101-116.
62. Sanford NN, Sher DJ, Ahn C, et al. Prevalence and nondisclosure of complementary and alternative medicine use in patients with cancer and cancer survivors in the United States. JAMA Oncol. 2019;5(5):735-737.
63. Kavookjian J, Wittayanukorn S. Interventions for adherence with oral chemotherapy in hematological malignancies: a systematic review. Res Social Adm Pharm. 2015;11(3):303-314.
64. Weingart SN, Toro J, Spencer J, et al. Medication errors involving oral chemotherapy. Cancer. 2010;116(10):2455-2464.
65. Monestime S, Page R, Jordan WM, et al. Prevalence and predictors of patients reporting adverse drug reactions to health care providers during oral targeted cancer treatment. J Am Pharm Assoc (2003). 2021;61(1):53-59.
The content contained in this article is for informational purposes only. The content is not intended to be a substitute for professional advice. Reliance on any information provided in this article is solely at your own risk.
To comment on this article, contact rdavidson@uspharmacist.com.






