USPharm. 2021:46(9)26-33.
ABSTRACT: Irritable bowel syndrome with predominant constipation (IBS-C) is a chronic disorder that primarily affects women. In 2020, the first clinical guideline by the American College of Gastroenterology for the treatment of IBS was published. A variety of nonpharmacologic recommendations for IBS-C include gut-directed psychotherapy, psyllium fiber, peppermint oil, and a diet low in fermentable oligosaccharides, disaccharides, monosaccharides, and polyols. Women with IBS-C have four FDA-approved prescription-treatment options including lubiprostone, linaclotide, plecanatide, and tegaserod. Selection of medication therapy may be guided by cost/insurance coverage, adverse-effect profile, and desired frequency of dosing.
Irritable bowel syndrome (IBS) is a chronic disorder (one of the many known functional gastrointestinal disorders) of gut-brain interaction that may negatively impact patients’ quality of life. This syndrome is characterized by recurrent abdominal pain associated with a change in frequency or form of bowel habits.1,2 IBS is currently divided into four subtypes based on the most prominent bowel habit: IBS with predominant constipation (IBS-C), IBS with predominant diarrhea, IBS with mixed bowel habits, and IBS unclassified.3 IBS predominantly affects women at a ratio of 2:1, with female patients presenting with more constipation and abdominal pain, rather than diarrhea.4 A population-based survey completed in the United States, Canada, and United Kingdom revealed a 4.4% to 4.8% prevalence of IBS among responders.5 The American College of Gastroenterology published guidelines in 2020 outlining the diagnosis and management of IBS. This article will focus on IBS-C in women and the associated pharmacologic options.
The pathophysiology of IBS is complex and not fully understood, with diet, gut, psychological, genetic, and central factors all potentially contributing to the disorder. However, each of these is not always present in every patient.4 Women commonly encounter conditions that overlap with IBS, likely leading to the increased prevalence in this population. Some examples of these overlap syndromes are fibromyalgia, migraine headache, and chronic fatigue syndrome. Additionally, there are gynecologic conditions that have been seen to co-occur with IBS including endometriosis, dysmenorrhea, and chronic pelvic pain.4 Hormones are involved in gastrointestinal (GI) dysfunction, and the overexpression of progesterone receptors in the intestines may play a role in altering GI transit leading to constipation.6
Diagnostic Criteria
IBS-C is diagnosed using a symptom-based approach. The Rome IV diagnostic criteria are the gold standard for diagnosing IBS-C. These criteria include recurrent abdominal pain on average at least 1 day per week in the last 3 months, associated with two or more of the following: related to defecation, associated with a change in stool frequency, and/or associated with a change in appearance of stool.3 Distinct from IBS-C, functional constipation is a disorder where the patient has infrequent stools or difficulty defecating, but abdominal pain and bloating are not the predominant symptoms, as they are in IBS-C.3 The above criteria must be filled for at least 3 months with symptom onset being at least 6 months prior to diagnosis. Additionally, to be subtyped as IBS-C, patients will have hard or lumpy stools greater than 25% of the time and mushy or watery stools less than 25% of the time.3 Having patients keep a daily diary of their bowel habits for 2 weeks can be helpful in determining the subtype diagnosis of IBS.
While this symptom-based diagnostic strategy is recommended, further testing, such as a colonoscopy, should be considered in patients with alarm features.1 Alarm features include unintentional weight loss, hematochezia, melena, onset after age 50 years, family history of irritable bowel disease, colon cancer, or other significant GI disease.1,2
An emphasis on a diagnostic strategy based upon the Rome IV criteria rather than a strategy of exclusion is beneficial to cutting overall costs to patients. Patients with IBS-C, when compared with other IBS subtypes, have the highest costs related to diagnostic testing that is unnecessary, in most cases.1 Additional diagnostic testing in patients without alarm symptoms has shown a low diagnostic yield and does not improve patient outcomes.1 Through the use of the Rome IV criteria and a clinical history, IBS-C can be diagnosed without patients incurring unnecessary healthcare costs. Another benefit of this diagnostic approach is that it shortens time to initiation of therapy.1 By properly treating patients sooner they will have a quicker improvement of IBS symptoms resulting in less time off from work.2
Nonpharmacologic Therapy
Since IBS is a disorder of the gut-brain interaction, gut-directed psychotherapies (GDPs) including cognitive-behavior therapy–GI and gut-directed hypnotherapy are recommended. These therapies can help improve the severity of symptoms by targeting cognitive and affective factors such as fear of symptoms, stress sensitivity, pain catastrophizing, hypervigilance, and somatization.1 GDPs encompass a range of skill-based techniques consisting of relaxation training, reframing of unhelpful thoughts, decreasing helplessness, exposure, and behavioral experimentation around avoidance of symptoms.1,7 The GDPs should be used in conjunction with pharmacotherapy.1
Dietary fiber can improve stool frequency and viscosity and is generally safe, thereby providing an inexpensive first-line option for patients.1 It is important that the fiber is not soluble and is poorly fermentable to provide benefit for IBS-C patients. This type of fiber exerts a laxative effect to help with constipation, whereas fiber that is fermentable produces gas that would further aggravate any symptoms of bloating and flatulence.8 Psyllium is an example of a soluble, poorly fermentable fiber that has shown improvement of symptoms.1
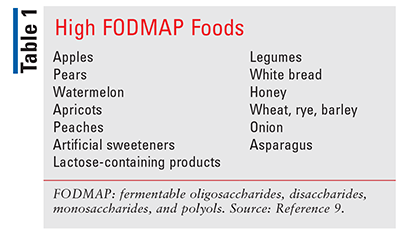
Another dietary option to help improve general symptoms of IBS is a low fermentable oligosaccharides, disaccharides, monosaccharides, and polyols (FODMAPs) diet. Foods high in FODMAPs lead to increased fermentation in the colon, producing short-chain fatty acids and gases and can trigger meal-related symptoms in those with IBS.1 Examples can be found in TABLE 1.9 Due to the low quality of evidence and the risk for nutritional deficiencies, the guideline from the American College of Gastroenterology suggests only a limited trial under supervision of a dietician.1
Peppermint oil has some evidence to show it can help relieve abdominal pain in IBS patients. Peppermint oil has multiple properties that can benefit patients such as antispasmodic, anti-inflammatory, antimicrobial, anesthetic, immunomodulating, and antioxidant effects.10 Enteric-coated preparations used on a continuous basis can offer benefit while minimizing adverse effects, such as heartburn.1
While the complementary therapies outlined above can be used to aid in the treatment of general symptoms of IBS-C, probiotics and polyethylene glycol (PEG) are not recommended.1 PEG has been shown to improve bowel symptoms but does not alleviate abdominal pain, and therefore should not be used alone to treat IBS-C.1 Studies analyzing the use of probiotics in IBS have been inconsistent in showing benefit to individual symptoms, and used multiple types of probiotics, and for this reason they are not currently recommended.1
Pharmacologic Therapy
Three secretagogues are strongly recommended to treat the global symptoms of IBS-C, lubiprostone (Amitiza), linaclotide (Linzess), and plecanatide (Trulance).1 The most recent guideline does not include a recommendation for which secretagogue to use first. If the secretagogues do not provide an adequate response, tegaserod (Zelnorm) is a second-line option for women younger than age 65 years. The pharmacology of these agents is outlined below; dosing and contraindications can be found in TABLE 2. When selecting an initial medication, the patient and provider may want to evaluate the options based on their adverse-effect profile, frequency and convenience of dosing, cost, and the provider’s clinical experience.
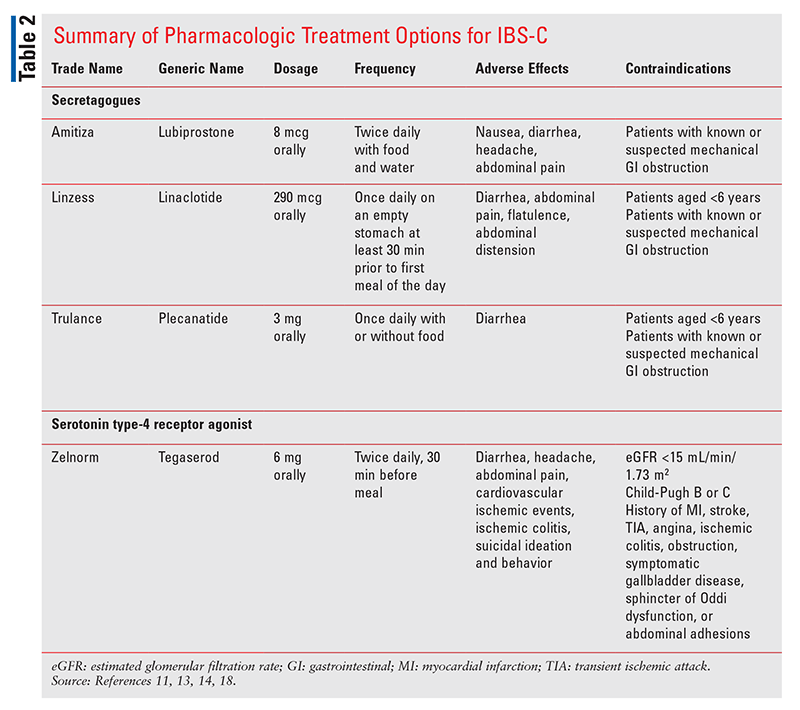
Lubiprostone is FDA approved for adult women with IBS-C. Lubiprostone is a prostaglandin E1 analog that activates chloride channels on intestinal epithelial cells. Intestinal secretion and peristalsis are increased through activation of these chloride channels; animal studies have suggested it may restore barrier function in those with increased intestinal permeability.1 The drug is 94% protein bound and is not metabolized via the cytochrome P450 (CYP450) system with no clinically significant drug interactions anticipated.11 A combined analysis of two identically designed phase III trials with 91.6% of participants female showed statistical separation between treatment and placebo groups after 2 months.12 The primary end point, moderate relief of symptoms for 4 of 4 weeks or significant relief for 2 of 3 months, was achieved by 17.9% and 10.1% of participants in the lubiprostone and placebo groups, respectively.12 Not only was the primary end point statistically significant (P = .001) but the following secondary end points also showed significant improvement: abdominal pain and discomfort, bloating, straining and severity of constipation, increased bowel movement frequency, and stool consistency.12
Linaclotide and plecanatide are guanylate cyclase activators that are FDA approved for the treatment of IBS-C. Guanylate cyclase-C (GC-C) receptors are in the apical membrane of intestinal epithelial cells, and their activation leads to an increase in secretion of intestinal fluid and peristalsis as well as reduced activation of visceral nociceptive neurons. Both linaclotide and plecanatide are metabolized within the GI tract to active metabolites, and no drug interactions through CYP450 enzymes or common transporters are anticipated.13,14 These are both well tolerated and comparable in efficacy.
A double-blind, randomized 26-week trial of linaclotide showed statistical significance when compared with placebo (P <.0001) in responders to the FDA end point.15 A responder to the FDA end point is defined as a patient who met both of the following criteria in the same week for at least 6 out of the first 12 weeks of the treatment period: 1) an improvement of ≥30% from baseline in the average of the daily worst abdominal pain scores and 2) an increase of ≥1 complete spontaneous bowel movement from baseline.15 Over the first 12 weeks of treatment with linaclotide, 33.7% of patients were FDA end point responders compared with just 13.9% of patients in the placebo group (P <.0001). In the analysis of the full 26-week treatment period, the significant response in the linaclotide group was maintained with 32.4% responders and only 13.2% in the placebo group.15
A meta-analysis of plecanatide using the same FDA end point responder showed an odds ratio of response to treatment of 1.87 (95% CI, 1.47-2.38; number needed to treat = 9) when compared with placebo.16 Additionally, the GC-C agonists provide improvement in symptoms of discomfort, fullness, cramping, and bloating, and a clinically meaningful improvement in their IBS quality-of-life score.15,17 These GC-C agonists provide a relatively quick response, with improvement in as little as 1 week that is maintained over time.1
Although tegaserod in an option for women younger than age 65 years, it is contraindicated in women with more than one cardiovascular (CV) risk factor (TABLE 3). Tegaserod is a serotonin type-4 receptor agonist that accelerates GI transit by initiating the peristaltic reflex.1 Agonism at this serotonin receptor has also been seen to reduce visceral hypersensitivity in IBS patients.1 Tegaserod is approximately 98% protein bound and is metabolized via hydrolysis in the stomach with about two-thirds being excreted unchanged in the feces.18
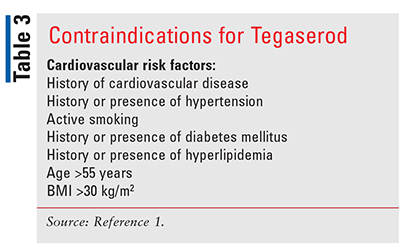
One study that led to the initial approval of tegaserod in 2002 was a 12-week, double-blind, placebo-controlled trial that consisted of 83% female patients. The primary end point of the trial was the Subject’s Global Assessment (SGA) of Relief with an effect seen as early as 1 week.19 In participants receiving tegaserod, 50% achieved response compared with just 36.6% of those receiving placebo (P <.05). In another all-female, 12-week study, the SGA of Relief end point was met by 43.5% in the tegaserod group compared with 38.8% of those on placebo (P <.033).20 Additionally, both studies showed significant improvement in abdominal pain, stool consistency, and stool frequency. While tegaserod was voluntarily removed from the market in 2007 due to concerns about CV events, it was approved again in 2019. Two external adjudications that evaluated 18,645 patients to assess the risk of CV event were performed before it was returned to markets. While this process showed 13 patients (treated with tegaserod) with confirmed CV events, only 1 CV event and no major CV events were found in women younger than age 65 years without a history of CV ischemic disease and one or fewer cardiac risk factors.1 Thus this medication is now approved for women younger than age 65 years with ≤1 CV risk factor. Tegaserod is a viable option with a unique mechanism of action for those who do not find benefit from secretagogues and fit the criteria above. It is important to note that if adequate symptom control is not achieved after 4 to 6 weeks, tegaserod should be discontinued.18
The guidelines also discuss tricyclic antidepressants (TCAs) as another pharmacologic option to help alleviate general IBS symptoms (not specific to constipation subtype). TCAs are considered neuromodulators that can treat abdominal pain by improving visceral and central pain as well as improving coexisting psychological distress patients may experience. An important consideration with their use in IBS-C is the potential for TCAs to cause constipation. Starting at a low dose, for example, 10 mg amitriptyline, with a gradual dose titration to achieve relief while minimizing side effects is recommended.1
Cost and Affordability of Medications
While prescription pharmacologic therapy is the mainstay of treatment for IBS-C, these medications come with a hefty price tag. Optimizing first-line nonpharmacologic and alternative treatment options may help prevent patients from needing to use the expensive prescription medications. Psyllium fiber is both an inexpensive and easily accessible OTC recommendation to use initially in patients with IBS-C as it improves overall symptoms. While a trial of the low FODMAP diet may incur the initial added cost of a dietician, patients who have symptom improvement from the diet can be identified within 2 to 6 weeks.1 Those who don’t see an improvement in abdominal pain and bloating during this limited trial can move on to other treatment modalities, whereas patients that do respond can continue to work with their dietitian and potentially lower their overall medication cost long-term. PEG could be considered as well, given that it is inexpensive and improves stool frequency and consistency. In those looking to minimize cost, PEG could be used for its laxative effect in combination with other approaches, such as peppermint oil and GDPs, to help with abdominal pain.
The average wholesale prices for 1 month of therapy for lubiprostone, linaclotide, plecanatide, and tegaserod are approximately $400, $560, $565, and $515, respectively, leading to an even higher cost for patients.21-24 Since there is no clear recommendation for which secretagogue to pick, a consideration of affordability and insurance coverage for the patient can help guide a decision on which medication to initiate. Lubiprostone is the only option that currently has a generic available on the market and, as a result, will likely have better insurance coverage in the future. For women with commercial insurance, Linzess and Trulance have copay cards for a $30 and $25, 90-day supply, respectively, making these options significantly more affordable. These three first-line options also have patient-assistance programs that may offer the medications free if the patient qualifies. For women who do not find benefit from the secretagogues, tegaserod has a copay card bringing the cost down to as little as $25 per month.
Conclusion
IBS-C affects predominantly female patients and is a chronic illness that can negatively impact quality of life. There are many nonpharmacologic and pharmacologic treatment options to help sufferers achieve symptom improvement and/or relief. The prescription options are well tested in the female population with some of the options showing a quick and sustained symptom response. Patients have multiple options if an initial drug choice does not provide relief, as there are three different mechanisms of action between the four prescription drugs.
The content contained in this article is for informational purposes only. The content is not intended to be a substitute for professional advice. Reliance on any information provided in this article is solely at your own risk.
REFERENCES
1. Lacy BE, Pimentel M, Brenner DM, et al. ACG clinical guideline: management of irritable bowel syndrome. Am J Gastroenterol. 2021;116(1):17-44.
2. Farmer AD, Wood E, Ruffle JK. An approach to the care of patients with irritable bowel syndrome. CMAJ. 2020;192(11):E275-E282.
3. Mearin F, Lacy BE, Chang L, et al. Bowel disorders . Gastroenterology. 2016;S0016-5085(16)00222-5.
4. Harris LA, Umar SB, Baffy N, Heitkemper MM. Irritable bowel syndrome and female patients. Gastroenterol Clin North Am. 2016;45(2):179-204.
5. Palsson OS, Whitehead W, Tornblom H, et al. Prevalence of Rome IV functional bowel disorders among adults in the United States, Canada, and the United Kingdom. Gastroenterology. 2020;158:1262-1273.
6. Roisinblit KC. Irritable bowel syndrome in women. J Midwifery Womens Health. 2013;58(1):15-24.
7. Black CJ, Thakur ER, Houghton LA, et al. Effect of psychological therapies, for irritable bowel syndrome systematic review and network meta-analysis. Gut. 2020;69:1441-1451.
8. Moayyedi P, Quigley EM, Lacy BE, et al. The effect of fiber supplementation on irritable bowel syndrome: a systematic review and meta-analysis. Am J Gastroenterol. 2014;109(9):1367-1374.
9. Böhn L, Störsrud S, Liljebo T, et al. Diet low in FODMAPs reduces symptoms of irritable bowel syndrome as well as traditional dietary advice: a randomized controlled trial. Gastroenterology. 2015;149(6):1399-1407.e2.
10. Alammar N, Wang L, Saberi B, et al. The impact of peppermint oil on the irritable bowel syndrome: a meta-analysis of the pooled clinical data. BMC Complement Altern Med. 2019;19(1):21.
11. Amitiza. Package insert. Bethesda, MD: Sucampo Pharma Americas, Inc; 2012.
12. Drossman DA, Chey WD, Johanson JF, et al. Clinical trial: lubiprostone in patients with constipation-associated irritable bowel syndrome-—results of two randomized, placebo-controlled studies. Aliment Pharmacol Ther. 2009;29(3):329-341.
13. Linzess. Package insert. Boston, MA: Ironwood Pharmaceuticals, Inc; 2021.
14. Trulance. Package insert. Bridgewater, NJ: Salix Pharmaceuticals, Inc; 2021.
15. Chey WD, Lembo AJ, Lavins BJ, et al. Linaclotide for irritable bowel syndrome with constipation: a 26-week, randomized, double-blind, placebo-controlled trial to evaluate efficacy and safety. Am J Gastroenterol. 2012;107(11):1702-1712.
16. Shah ED, Kim HM, Schoenfeld P. Efficacy and tolerability of guanylate cyclase-c agonists for irritable bowel syndrome with constipation and chronic idiopathic constipation: a systematic review and meta-analysis. Am J Gastroenterol. 2018;113(3):329-338.
17. Atluri DK, Chandar AK, Bharucha AE, Falck-Ytter Y. Effect of linaclotide in irritable bowel syndrome with constipation (IBS-C): a systematic review and meta-analysis. Neurogastroenterol Motil. 2014;26(4):499-509.
18. Zelnorm. Package insert. Louisville, KY: Sloan Pharma S.a.r.l.; 2019.
19. Müller-Lissner SA, Fumagalli I, Bardhan KD, et al. Tegaserod, a 5-HT(4) receptor partial agonist, relieves symptoms in irritable bowel syndrome patients with abdominal pain, bloating and constipation. Aliment Pharmacol Ther. 2001;15(10):1655-1666.
20. Novick J, Miner P, Krause R, et al. A randomized, double-blind, placebo-controlled trial of tegaserod in female patients suffering from irritable bowel syndrome with constipation. Aliment Pharmacol Ther. 2002;16(11):1877-1888.
21. Lubiprostone. In: Lexi-Drugs. Hudson, OH: Lexi-Comp, Inc. Updated May 8, 2021. https://online.lexi.com.cuhsl.creighton.edu/lco/action/doc/retrieve/docid/patch_f/486290?cesid=751dELz43no&searchUrl=%2Flco%2Faction%2Fsearch%3Fq%3Damitiza%26t%3Dname%26va%3Damitiza. Accessed July 2, 2021.
22. Linaclotide. In: Lexi-Drugs. Hudson, OH: Lexi-Comp, Inc. Updated June 2, 2021. https://online.lexi.com.cuhsl.creighton.edu/lco/action/doc/retrieve/docid/patch_f/3885203?cesid=2ryddObnd2F&searchUrl=%2Flco%2Faction%2Fsearch%3Fq%3Dlinzess%26t%3Dname%26va%3Dlinzess. Accessed July 2, 2021.
23. Plecanatide. In: Lexi-Drugs. Hudson, OH: Lexi-Comp, Inc. Updated June 28, 2021. https://online.lexi.com.cuhsl.creighton.edu/lco/action/doc/retrieve/docid/patch_f/6433522?cesid=7lVCtu0ZZbU&searchUrl=%2Flco%2Faction%2Fsearch%3Fq%3Dtrulance%26t%3Dname%26va%3Dtrulance. Accessed July 2, 2021.
24. Tegaserod. In: Lexi-Drugs. Hudson, OH: Lexi-Comp, Inc. Updated April 19, 2021. https://online.lexi.com.cuhsl.creighton.edu/lco/action/doc/retrieve/docid/patch_f/7727?cesid=321eeeH89OD&searchUrl=%2Flco%2Faction%2Fsearch%3Fq%3Dzelnorm%26t%3Dname%26va%3Dzelnorm. Accessed July 2, 2021.
To comment on this article, contact rdavidson@uspharmacist.com.





