US Pharm. 2008;33(3):66-71.
Attention-deficit
hyperactivity disorder (ADHD) is characterized by inattentiveness,
impulsivity, and/or hyperactivity and represents a common behavioral disorder
of childhood. ADHD is defined by the Diagnostic and Statistical Manual of
Mental Disorders, Fourth Edition (DSM-IV) as consisting of at least six of
nine symptoms of inattention or hyperactivity/impulsivity for six or more
months that starts before the age of 7, with impairment in two†or more
settings, and is not caused by another mental disorder. Additionally, there
must be clear evidence of clinically significant impairment in social,
academic, or occupational functioning.1
A recent study found that 8.7%
of U.S. children aged 8 to 15, an estimated 2.4 million, met DSM-IV
criteria for ADHD.2 Previous studies have found persistence of ADHD
into adolescence to be 60% to 80%.3,4 Without effective treatment,
the short- and long-term consequences of ADHD include poor performance at
school and later at work, poor self-esteem, relationship problems, and
substance abuse.3
Treatment can include
behavioral therapy alone for milder ADHD or for preschool-aged children.5
Behavioral therapy alone, however, is not as effective in reducing symptoms
in patients with comorbid anxiety disorder or comorbid disruptive behavior. In
these patients, a combination of behavioral therapy and pharmacological
therapy should be used.6 The goals of therapy are to improve
relationships with family, teachers, and peers; decrease disruptive behaviors;
improve academic performance; increase independence in self-care; and increase
self-esteem.7
Stimulants
Stimulant
medications are first line in the pharmacologic treatment of ADHD and are
effective in 70% to 80% of children.8,9 They have been used for
many years; however, in the past they were only available as immediate-release
oral formulations, which required multiple daily doses. This did not allow for
a steady serum drug concentration throughout the day and was inconvenient. New
formulations allowing for once-daily dosing were developed; however, the
initial extended-release products (i.e., Ritalin SR, Metadate ER,
and Methylin ER) lasted for only six to eight hours. They would
cover the school day, but some children would still require a midday dose to
cover the late afternoon and early evening.
Since 2000, additional
products have been developed to provide a longer duration of action. The
longer-acting preparations and delivery systems allow for once-daily dosing
and eliminate the need for medication at school. These advances include an
osmotically controlled-release drug-delivery system; beaded delivery systems
that allow for biphasic drug release; a longer-acting molecule (atomoxetine);
transdermal patch (methylphenidate transdermal system); and a conjugated d
-amphetamine molecule (lisdexamfetamine). The two most recently approved
stimulants for ADHD are the methylphenidate transdermal system and
lisdexamfetamine. A comparison of currently available intermediate and
long-acting stimulant medications is summarized in TABLE 1.
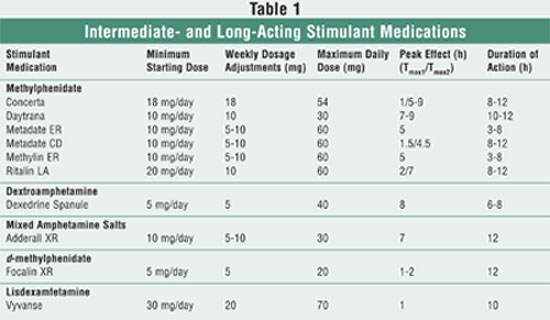
Methylphenidate Transdermal
System
The mechanism of
action for methylphenidate is believed to be the inhibition of dopamine and
norepinephrine reuptake in the presynaptic neuron.10 Daytrana is a
transdermal system that contains methylphenidate in a multipolymeric adhesive
matrix. This delivery system allows for identical composition per unit area of
all dosage strengths. After the patch is applied, it takes an average of 3.1
hours (range 1-6 h) before methylphenidate is detected in the plasma. When
applied to inflamed skin or if heat is applied to the patch, the rate and
extent of absorption are increased. Plasma concentrations peak after about
seven to nine hours. The mean elimination half-life of d-
methylphenidate (the more active enantiomer) after removal of the transdermal
system is approximately three to four hours.11
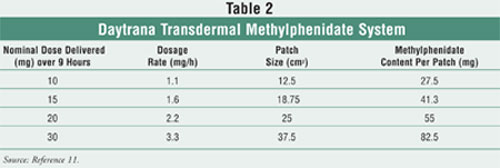
Daytrana is approved for
children 6 to 12 years of age, and four dosage strengths, listed in TABLE 2
, are available.11 Unlike oral extended-release products that have
an immediate-release phase followed by an extended-release phase, the dose for
the methylphenidate transdermal system is consistent throughout the dosing
period. When transitioning from oral methylphenidate to the transdermal
system, the lowest dosage strength should be used (10 mg q 9 h) and then
titrated upward. Since the transdermal system avoids first-pass metabolism,
there is a difference in bioavailability compared to the oral products. The
transdermal system should be worn on the hip, alternating hips and application
sites. If possible, care should be given to avoid the waistline since clothes
can cause the patch to rub off. The patch should be used immediately after
removal from the pouch and should be held in place for 30 seconds.11
A five-week randomized,
double-blind, multicenter, parallel-group, placebo-controlled,
dose-optimization study evaluated the safety and efficacy (the change in the
Spadafore Attention Deficit Hyperactivity Disorder Rating Scale [S-ADHD-RS-IV]
score from baseline) of methylphenidate transdermal system patches compared to
placebo, and with reference to Concerta. The main secondary outcome was to
assess the efficacy of the methylphenidate transdermal system using the
Conners' Teacher Rating Scale-Revised: Short Form (CTRS-R) in an academic
setting. Two hundred and seventy subjects were included in the ITT analysis.
The doses of the methylphenidate transdermal system and Concerta were titrated
up at weekly intervals until the optimal dose was achieved (based on
tolerability and effectiveness). Study analysis found that there was a
statistically significant difference in the ADHD-RS-IV score from baseline to
the end of the study in the methylphenidate transdermal system group compared
to placebo. There was also a statistically significant difference in the
CTRS-R score from baseline to the end of the study for the methylphenidate
transdermal system compared to placebo. Additional secondary objectives were
also studied, and a statistically significant improvement in scores was
demonstrated in the methylphenidate transdermal system compared to placebo.
12 Additional studies have been performed and have found similar results
to the above study.
In a long-term, open-label
study treating patients with the methylphenidate transdermal system for 12
hours, the most frequent adverse events were anorexia (46%), insomnia (30%),
viral infection (28%), and headache (28%). Twenty-four percent of subjects
were withdrawn from the study because of treatment-related adverse events.
These included application-site reaction (6%), anorexia (4%), and insomnia
(4%).11
Erythema is commonly seen with
the use of the methylphenidate transdermal system and should not be a reason
for discontinuation. Alternating patch sites and hips will help to minimize
this erythema. Contact sensitization, however, has been reported, and the
transdermal system should be removed and discontinued if it is suspected.
Signs of sensitization include erythema accompanied by a more intense local
reaction (edema, papules, vesicles) that does not resolve in 48 hours or
extends outside of the patch site. Patients who are sensitized from use of the
methylphenidate transdermal system may develop systemic sensitization or other
systemic reactions if methylphenidate-containing products are taken orally.
11
Like other methylphenidate
products, the transdermal system should not be used in patients with marked
anxiety, tension and agitation, glaucoma, motor tics, or a family history or
diagnosis of Tourette's syndrome. Additionally, it should be avoided in
patients receiving monoamine oxidase (MAO) inhibitors or in anyone with a
hypersensitivity to methylphenidate or components of the transdermal system.
11
Sudden death has been reported
in patients with structural cardiac abnormalities or other heart problems
receiving CNS stimulant treatment at usual doses. Therefore, stimulant
medications should generally not be used in these patients. Since stimulant
medications can cause an increase in blood pressure and heart rate, caution
should be used in treating patients whose underlying condition may be
compromised by increases in those variables. In patients with a pre-existing
psychotic disorder, administration of stimulants may worsen symptoms of
behavior disturbance and thought disorder. Treatment has been reported to
infrequently cause an emergence of new psychotic or manic symptoms.
Additionally, caution should be taken in using stimulants in patients with
bipolar disorder. Weight and height should be monitored periodically since
stimulants can cause long-term suppression of growth. Stimulants may also
lower the seizure threshold. 11
The methylphenidate
transdermal system offers several advantages over oral methylphenidate. The
most obvious advantage is for patients who have trouble swallowing pills or
capsules. Since this new method of administration does not undergo first-pass
metabolism, parents will no longer need to worry about the effect of food on
the rate and the extent of absorption of the medication.11
Additionally, the transdermal system will eliminate any fear of children
accidentally chewing up the extended-release beads.
Unlike previous oral
methylphenidate formulations that included only dosage titration, the
methylphenidate transdermal system offers both a dosage titration by
increasing the patch size (increase in mg/h) in addition to duration of patch
wear. If patients experience adverse effects before the end of the nine-hour
wear period, the patch can be discontinued early. A further advantage includes
the possible application of the transdermal system before a child awakens to
allow for early-morning coverage.13
In a recent study, Arnold et
al discussed not only wearing the patch for fewer than nine hours, but also
wearing it for up to 16 hours if additional coverage is needed.13
Although this may be a potential future option, caution should be used in
wearing it beyond the nine-hour time period. The original manufacture studies
for the transdermal system were for a total wear time of 12 hours; however,
due to an increase in adverse effects (anorexia, insomnia, and weight loss)
the recommended wear time was reduced to nine hours.12
It has been hypothesized that
the patch will deter adolescents from trying to abuse it. Once the patch has
been worn and then taken off, it is difficult to reapply. Additionally, the
only way to extract the methylphenidate from the transdermal system is by a
chemical process.12
Lisdexamfetamine
Although the
mechanism of action of lisdexamfetamine is unknown, it is believed to inhibit
the reuptake of dopamine and norepinephrine in the presynaptic neuron.14
Vyvanse is an oral stimulant that is a prodrug of dextroamphetamine
(Dexedrine). Lisdexamfetamine is converted to d-amphetamine and L
-lysine when it undergoes first-pass metabolism. Plasma concentrations for
lisdexamfetamine and d-amphetamine peak after one hour and 3.5 hours,
respectively. A meal high in fat will prolong the plasma concentration of d
-amphetamine by about one hour. 14
Vyvanse is approved for the
treatment of ADHD in patients 6 to 12 years. The starting dose is 30 mg/day
given in the morning for all patients, regardless of previous stimulant
medication regimen. The recommended dosage increase is 20 mg/day at weekly
intervals, with a maximum daily dose of 70 mg/day. Lisdexamfetamine may be
taken without regard to food and should not be taken in the afternoon due to
the possibility of insomnia. The capsule may be taken whole or dissolved in a
glass of water and taken immediately.14
A multicenter, randomized,
double-blind, forced-dose, parallel-group study was conducted in 290 patients.
Patients received either lisdexamfetamine 30, 50, or 70 mg or placebo for four
weeks. The primary efficacy outcome was the change from baseline in the
ADHD-RS-IV score. Secondary efficacy outcomes included additional scales
that assessed improvement in ADHD. The study found that each of the doses
studied produced statistically significant improvements in all of the outcomes
when compared to placebo.15 A second study also found significantly
improved scores on multiple scales (including a scale to measure classroom
manifestations of ADHD, a math scale, and a global improvement scale).16
The most common adverse
effects during a four-week premarketing clinical trial included decreased
appetite (39%), insomnia (19%), upper gastrointestinal abdominal pain (12%),
headache (12%), and irritability (10%). Additionally, vomiting (9%), decreased
weight (9%), nausea (6%), dry mouth (5%), dizziness (5%), affect lability
(3%), rash (3%), somnolence (2%), and tic (2%) were reported. Ten percent of
patients receiving lisdexamfetamine discontinued treatment due to adverse
events. These included ventricular hypertrophy, tic, vomiting, psychomotor
hyperactivity, insomnia, and rash.14
The warnings and precautions
are the same as those for the methylphenidate transdermal system and are
listed above.
Several drug interactions
exist with lisdexamfetamine. Amphetamines may enhance the activity of
tricyclic antidepressants (TCAs), and desipramine, protriptyline, and possibly
other TCAs can cause increased d-amphetamine in the brain, leading to
cardiovascular effects. MAO inhibitors slow amphetamine metabolism, which can
cause headaches, other signs of hypertensive crisis, or malignant
hyperpyrexia. Amphetamines may antagonize the hypotensive effects of
antihypertensives and inhibit adrenergic blockers. Haloperidol and lithium
carbonate inhibit the central stimulant effects of amphetamines. Amphetamines
may delay intestinal absorption of phenobarbital and phenytoin.14
One advantage of
lisdexamfetamine is that the L-lysine has to be cleaved off once it reaches
the gastrointestinal tract, so the risk for abuse is thought to be much lower
than that of immediate-release formulations and formulations that have a
quicker onset. A study was conducted in patients with a history of drug abuse
to try to determine the abuse potential of lisdexamfetamine. The subjects
received lisdexamfetamine 100 mg or IR d-amphetamine 40 mg, and
subjective effects were measured on a scale of "Drug Liking Effects,"
"Amphetamine Effects," and "Stimulant Effects." These effects were seen in
significantly fewer patients receiving lisdexamfetamine than with IR d
-amphetamine. However, lisdexamfetamine 150 mg produced increases in positive
subjective responses that were similar to that of d-amphetamine 40 mg
and diethylpropion 200 mg. Based on these results, the abuse potential may
increase as dosage increases.14
Conclusion
The development of
new stimulant formulations continue to help patients with ADHD find a
treatment regimen that works for them. The methylphenidate transdermal patch
has changed the management of ADHD by allowing both a titration in dose and a
variation in the duration of action. This new route of administration also
offers many additional advantages. Lisdexamfetamine is the first stimulant
prodrug to be developed and will hopefully have limited abuse potential.
REFERENCES
1. Disorders
usually first diagnosed in infancy, childhood, or adolescence. Diagnostic
and Statistical Manual of Mental Disorders DSM-IV, Fourth Edition-Text
Revision. Washington, DC: American Psychiatric Association; 2002.
2. Froehlich TE,
Lanphear BP, Epstein JN, et al. Prevalence, recognition, and treatment of
attention-deficit/hyperactivity disorder in a national sample of US children.
Arch Pediatr Adolesc Med. 2007;161:857-864.
3. Barkley RA, Fischer M, Edelbrock CS, Smallish L. The adolescent outcome of hyperactive children diagnosed by research criteria: I: an 8-year prospective follow-up study. J Am Acad Child Adolesc Psychiatry. 1990;29:546-557.
4. Mannuzza S, Klein R, Bessler A, et al. Adult psychiatric status of hyperactive boys grown up. Am J Psychiatry. 1998;155:493-498.
5. Conners CK, March JS, Frances A, et al. The Expert Consensus Guideline Series: treatment of attention-deficit/hyperactivity disorder. J Attention Disord. 2001;4(suppl 1):7-128.
6. A 14-month randomized clinical trial of treatment strategies for attention-deficit/hyperactivity disorder. The MTA Cooperative Group. Multimodal Treatment Study of Children with ADHD. Arch Gen Psychiatry. 1999;56:1073-1086.
7. Clinical practice guideline: treatment of the school-aged child with attention-deficit/hyperactivity disorder. Pediatrics. 2001;108:1033-1044.
8. Greenhill LL, Swanson JM, Vitiello B, et al. Impairment and deportment responses to different methylphenidate doses in children with ADHD: the MTA titration trial. J Am Acad Child Adolesc Psychiatry. 2001;40:180-187.
9. Spencer T, Biederman J, Wilens T, et al. Pharmacotherapy of ADHD across the life cycle. J Am Acad Child Adolesc Psychiatry. 1996;35:409-432.
10. Challman TD, Lipsky JJ. Methylphenidate: its pharmacology and use. Mayo Clin Proc. 2000;75:711-721.
11. Daytrana [package insert]. Available at: www.daytrana.com/HCP/PDFs/DaytranaPrescribingInformation.pdf. Accessed January 1, 2008.
12. FDA Psychopharmacologic Drugs Advisory Committee, December 2, 2005, briefing information. Available at: www.fda.gov/ohrms/dockets/ac/05/briefing/2005-4195B-index-with-disclaimer.htm. Accessed December 13, 2007.
13. Arnold LE, Lindsay RL, Lopez FA, et al. Treating attention-deficit/hyperactivity disorder with a stimulant transdermal patch: the clinical art. Pediatrics. 2007;120:1100-1106.
14. Vyvanse [package insert]. Available at: www.vyvanse.com/pdf/prescribing_ information.pdf. Accessed January 1, 2008.
15. Biederman J, Krishnan S, Zhang Y, et al. Efficacy and tolerability of lisdexamfeteamine dimesylate (MRP-104) in children with attention-deficit/hyperactivity disorder: a phase III, multicenter, randomized, double-blind, forced-dose, parallel-group study. Clin Ther. 2007;29:450-463.
16. Biderman J, Boellner S,
Childress A, et al. Lisdexamfetamine dimesylate and mixed amphetamine salts
extended-release in children with ADHD: a double-blind, placebo-controlled,
crossover analog classroom study. Biol Psychiatry. 2007;62:970-976.
To comment on this article, contact
editor@uspharmacist.com.






