US Pharm. 2019;44(10):29-32.
ABSTRACT: In 1987, the American Diabetes Association first recommended that patients monitor their blood glucose. Since then, self-monitoring of blood glucose (SMBG) has become an integral part of diabetes management. Self-monitoring provides real-time data to influence medication selection, alert patients to hypoglycemia, and inspire lifestyle modifications to help diabetic patients achieve their A1C goal. Patients are often given insufficient training in the nuances of SMBG. Pharmacists can help diabetic patients fill this knowledge gap and unlock the full potential of SMBG by teaching them proper monitoring techniques, when to test, and how to use results.
The first diabetes blood glucose test strip was developed in 1965, but the first glucometer did not reach the market until 1970.1 The first glucometer, which was available for use only within the healthcare system, weighed 1.2 kg (about 2.5 lb). It was not until 1980, when a digital display was developed, that the glucometer was recommended for home use.1 At that time, glucometers were typically used only by patients with type 1 diabetes. In 1987, the American Diabetes Association (ADA) established guidelines for self-monitoring of blood glucose (SMBG).2 Since then, SMBG has become an integral part of diabetes management.2
Technology has resulted in improvements in glucometer functionality. Today’s glucometers are digital, fit in the palm of the hand, require a miniscule amount of blood, can connect to a computer or smartphone, and are used by nearly all diabetic patients. In the United States, 1.26 million glucometers were sold in 2018, a sizable increase from the 960,000 units sold in 2017.3 The pharmacy remains one of the most common places to purchase a glucometer, ideally positioning the community pharmacist for teaching diabetic patients how to test, when to test, and how to incorporate results into glucose pattern management (GPM).
How to Test
Traditionally, SMBG is performed on the fingertip at any location between the fingernail and the first knuckle of the finger that is most comfortable for the patient.4 General directions for SMBG are given in TABLE 1 (refer to the glucometer’s instruction manual for specific directions). To minimize the pain associated with fingertip testing, the patient should rotate testing sites and test on the side of the third, fourth, or fifth finger as opposed to the pad of the finger.2 Sore fingers are a common complaint among diabetic patients. In response to patient complaints, many glucometer manufacturers have calibrated devices to accurately detect blood glucose at alternative testing sites. Such sites include the forearm, upper arm, palm, thigh, calf, and base of the thumb. The meters differ in the site they can be used for, so the manufacturer’s instructions should be consulted. A 2003 study compared glucose monitoring via traditional fingertip testing and forearm testing.5 Of the 112 patients recruited, 71% stated that collection of blood from the forearm caused no pain or less pain than fingertip testing.5 Although alternative sites are less painful for some patients, they are not accurate when blood glucose is rapidly changing, such as within 2 hours of eating a meal, after insulin administration, during exercise, or when hypoglycemia is suspected.6
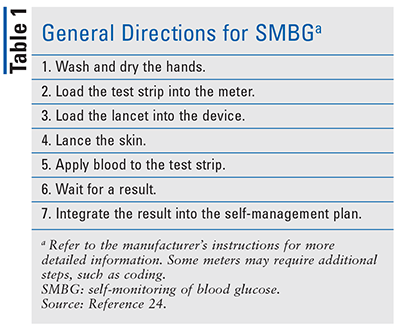
Appropriate blood-collection technique is critical for an accurate blood glucose result. The testing site must be washed with soap and water or alcohol and dried, as food residue can skew the results.2 A new, clean lancet should be used for each test; a lancet used more than once can become dull and cause greater pain when piercing the skin.2 Most devices offer a choice of lancing depth. To select an appropriate depth, the patient must apply the device firmly but without force to the testing site and keep the amount of pressure consistent.2 If the lancet does not pierce deeply enough and it is necessary to apply force to milk the blood from the testing site, the result could be inaccurate because the sample is diluted with interstitial fluid.4 If the lancet pierces too deeply, the patient will experience more pain and bleed for longer than necessary. Previously, it was common practice to wipe away the first drop of blood after lancing the skin because the first drop contains a high amount of platelets, which could clot the sample during reading. It is no longer necessary to do this because current blood glucose meters are fast enough to report results before clotting takes place.4
Continuous glucose monitoring (CGM) is another option for SMBG. With CGM, the patient wears a sensor that acts as a test strip. Attached to the sensor is a transmitter that sends the glucose data to the receiver (or reader). The receiver then tracks current and stored glucose readings.7 Not all CGM devices are equivalent: Some devices include alarms to alert the wearer of hypoglycemia or hyperglycemia, some require calibration as frequently as every 12 hours with standard fingertip testing, and some are available in combination with insulin pump therapy.8 CGM devices measure interstitial glucose rather than blood glucose. Interstitial glucose and blood glucose are not typically equal, and interstitial glucose values will lag behind blood glucose values.9 The lag time is important to keep in mind under conditions when the blood glucose is rapidly changing.9
When to Test
The most common times to perform SMBG are during a fasting state, before a meal, 1 to 2 hours after a meal, and at bedtime. Some patients may need to test at less common times, such as before snacks, before or after exercise, and prior to performing critical tasks.10 All patients should be educated to test blood glucose when hypoglycemia is suspected. Some signs of hypoglycemia are shakiness, irritability, confusion, tachycardia, and hunger.11 Determining when to test is an individualized process; however, some recommendations on SMBG are available for patients following one of these medication regimens: intensive insulin, basal insulin with or without other antidiabetic agents, or no insulin.
Intensive Insulin Regimen: The patients requiring most frequent SMBG are those who are following an intensive insulin regimen. An intensive insulin regimen is defined as insulin pump therapy or multiple insulin injections per day. The ADA recommends that these patients check blood glucose roughly six to 10 times per day at the following times: prior to meals and snacks, at bedtime, occasionally after meals, before exercise, when low blood glucose is suspected, after treating low blood glucose until normoglycemia is reached, and before critical tasks.10 The American Association of Clinical Endocrinologists (AACE) recommends that patients test four times per day and before any insulin injection, with more frequent checks after meals or in the middle of the night if the patient has frequent hypoglycemia or is not at targeted A1C.12 The information obtained from the different testing times will help guide insulin dosing and help minimize glycemic excursions. This patient population is most likely to benefit from using CGM to improve glycemic control, enhance quality of life, and minimize hypoglycemia.13,14
Basal Insulin With or Without Other Antidiabetic Agents: Patients taking basal insulin with or without other antidiabetic agents require significantly less intensive monitoring than patients on intensive insulin therapy. In patients using basal insulin, evidence is insufficient regarding when and how often to perform SMBG.10 However, fasting glucose values are most helpful to clinicians who are trying to titrate basal insulin. The AACE diabetes treatment guidelines include an algorithm to adjust basal insulin every 2 to 3 days based on fasting glucose readings in order to help the patient reach glycemic goals.12 Patients who are stable and are not adjusting their insulin doses require less intensive testing; however, the optimal strategy remains unknown.15 A study performed in the Netherlands divided stable patients into three groups, with SMBG performed once weekly, every 2 weeks, or every month. The researchers found no significant differences in glycemic control, quality of life, diabetes self-care activities, fasting blood glucose, or hypoglycemic events between the testing groups.16 Therefore, once a patient is stable on basal insulin and A1C is well controlled, testing frequency becomes less critical.
No Insulin: The benefit of SMBG in patients not on insulin therapy is under much debate. Neither the ADA nor the AACE can provide a recommendation as to when or how often a patient not taking insulin should test; however, both guidelines state that test results should be used to modify behavior or pharmacologic treatment in order to improve outcomes.10,12 In 2009, a meta-analysis found that the use of SMBG was associated with a reduction in A1C of 0.27% in patients with a baseline A1C of 8% to 10% and a 1.23% reduction in patients with a baseline A1C of >10%.17 A more recent meta-analysis agreed that SMBG would decrease A1C; however, subgroup analysis showed no significant difference in A1C reduction in patients who simply monitored blood glucose, but the difference was significant within the group of patients who monitored blood glucose and adjusted their diabetes management plan based on the results.18 Overall, recommendations to perform SMBG in patients not taking insulin must be individualized. The patients most likely to benefit from testing are those with an A1C >8% who are using the data to drive treatment decisions.
Glucose Pattern Management
GPM is a systematic approach to finding glycemic patterns in the SMBG data and then taking appropriate action based on those results.19 GPM starts with establishing glucose targets. General recommendations when targeting an A1C of <7% include targeting blood glucose between 80 and 130 mg/dL when fasting and <180 mg/dL 1 to 2 hours after the start of a meal.11 Once patients have goals in mind, they will accumulate several days of data to include not only blood glucose readings at various times but also food intake, physical activity, medication dosages, stress levels, and so on.20 Once the data have been collected, patients can analyze the information to find individual patterns and determine relationships between blood glucose and behavior.19,20 GPM helps patients understand how daily choices affect blood glucose results. Seeing the effects of diet and exercise on blood glucose can be a powerful motivator and reinforce successful behavior.21
Diet: When glucose patterns are being established, it is crucial to monitor food intake. Because studies on the ideal amount of carbohydrates to consume are inconclusive, patients should monitor carbohydrate intake in relation to glucose response to determine individual patterns.22 For example, suppose a patient has learned that a serving of rice raises his blood glucose. With continued testing, he determines that if his blood glucose is <120 mg/dL, he can safely eat a serving of rice at a meal without exceeding his postprandial goal. The patient also finds that if he wants to eat rice and his preprandial blood glucose is >120 mg/dL, he can eat only half a serving of rice to remain at goal. This is a prime example of how GPM in relation to dietary choices can be incorporated into a diabetes self-management plan.
Exercise: Exercise uses glucose as a form of energy and will result in lower SMBG readings. The ADA recommends 150 minutes of moderate- to vigorous-intensity physical activity per week plus two to three sessions of resistance training per week.22 Using exercise as a tool in GPM can help patients achieve maximal blood glucose control. For example, a patient who identified a pattern of hypoglycemia after exercising now eats a small snack prior to exercise and avoids the hypoglycemic event. This case illustrates how a patient was able to apply her glucose patterns to implement a self-care plan that caused her to remain within her blood glucose target. Patients commonly identify patterns of postprandial elevation. A recent study from China concluded that walking for 20 minutes after eating dinner can correct this pattern. The study found a significantly lower 2-hour postprandial glucose spike, peak glucose, and average glucose in patients who walked compared to those who did not.23 This information further proves the value of GPM in relation to exercise in influencing a diabetes self-management plan.
Conclusion
Performing SMBG alone does not improve diabetes outcomes; the result must be integrated into the patient’s clinical and self-management plans in order to provide benefit.10 Too frequently, patients are not given adequate education on SMBG, but pharmacists are in a prime position to help fill this educational void. Pharmacists can guide patients in how to use glucometers correctly and to test using proper techniques to obtain accurate results. They can also educate patients about important times to monitor their blood glucose, thus ensuring that the data obtained are useful for both the healthcare team and the self-management plan. Finally, pharmacists can help patients identify glucose patterns and develop actions they can take in response to trends. Because pharmacists rank among the most trusted and ethical healthcare professionals, their contribution to diabetes education can help patients unlock the full potential of blood glucose monitoring.
The views expressed herein are those of the author and do not reflect the official policy of the Department of the Army, the Department of Defense, or the U.S. Government.
REFERENCES
1. Clarke SF, Foster JR. A history of blood glucose meters and their role in self-monitoring of diabetes mellitus. Br J Biomed Sci. 2012;69(2):83-93.
2. Kirk JK, Stegner J. Self-monitoring of blood glucose: practical aspects. J Diabetes Sci Technol. 2010;4(2):435-439.
3. Statista. Sales of the leading blood-glucose meter brands in the United States in 2019 (in million U.S. dollars). www.statista.com/statistics/326855/blood-glucose-meter-brands-sales-in-the-us. Accessed September 20, 2019.
4. Dubois W. Blood glucose self-monitoring. Part 2: monitoring technique. Diabetes Self Manag. 2014;31(4):66-69.
5. Fedele D, Corsi A, Noacco C, et al. Alternative site blood glucose testing: a multicenter study. Diabetes Technol Ther. 2003;5(6):983-989.
6. Ellison JM, Stegmann JM, Colner SL, et al. Rapid changes in postprandial blood glucose produce concentration differences at finger, forearm, and thigh sampling sites. Diabetes Care. 2002;25(6):961-964.
7. Wahowiak L. Continuous glucose monitors. www.diabetesforecast.org/2017/mar-apr/images/2017-cg-cgm-infographic.gif. Accessed May 24, 2019.
8. Clinical Resource, continuous glucose monitoring FAQs. Pharmacist’s Letter/Prescriber’s Letter; February 2018.
9. Medtronic. Why sensor glucose does not equal blood glucose. www.medtronicdiabetes.com/customer-support/sensors-and-transmitters-support/why-sensor-glucose-does-not-equal-blood-glucose. Accessed September 13, 2019.
10. American Diabetes Association. 7. Diabetes technology: Standards of Medical Care in Diabetes—2019. Diabetes Care. 2019;42(suppl 1):S71-S80.
11. American Diabetes Association. 6. Glycemic targets: Standards of Medical Care in Diabetes—2018. Diabetes Care. 2018;41(suppl 1):S55-S64.
12. AACE Diabetes Research Center. Glycemic management in type 2 diabetes. http://outpatient.aace.com/type-2-diabetes/treatment. Accessed September 13, 2019.
13. Ruedy KJ, Parkin CG, Riddlesworth TD, et al. Continuous glucose monitoring in older adults with type 1 and type 2 diabetes using multiple daily injections of insulin: results from the DIAMOND trial. J Diabetes Sci Technol. 2017;11(6):1138-1146.
14. Grunberger G, Bailey T, Camacho PM, et al. Proceedings from the American Association of Clinical Endocrinologists and American College of Endocrinology consensus conference on glucose monitoring. Endocr Pract. 2015;21(5):522-533.
15. Hoffman RM, Shah JH, Wendel CS, et al. Evaluating once- and twice-daily self-monitored blood glucose testing strategies for stable insulin-treated patients with type 2 diabetes: the Diabetes Outcomes in Veterans Study. Diabetes Care. 2002;25(10):1744-1748.
16. Hortensius J, Kleefstra N, Landman GWD, et al. Effects of three frequencies of self-monitored blood glucose on HbA1c and quality of life in patients with type 2 diabetes with once daily insulin and stable control: a randomized trial. BMC Res Notes. 2018;11(1):26.
17. Poolsup N, Suksomboon N, Rattanasookchit S. Meta-analysis of the benefits of self-monitoring of blood glucose on glycemic control in type 2 diabetes patients: an update. Diabetes Technol Ther. 2009;11(12):775-784.
18. Hou YY, Li W, Qiu JB, Wang XH. Efficacy of blood glucose self-monitoring on glycemic control in patients with non-insulin-treated type 2 diabetes: a meta-analysis. Int J Nurs Sci. 2014;1(2):191-195.
19. Parkin CG, Davidson JA. Value of self-monitoring blood glucose pattern analysis in improving diabetes outcomes. J Diabetes Sci Technol. 2009;3(3):500-508.
20. Meece J. Pattern management: the key to excellent glycemic control. Scherer Clinical Communications. www.cecity.com/scherer/2014/pattern_management/scherer_pattern_management_print.pdf. Accessed September 13, 2019.
21. Powers MA, Davidson J, Bergenstal RM. Glucose pattern management teaches glycemia-related problem-solving skills in a diabetes self-management education program. Diabetes Spectrum. 2013;26(2):91-97.
22. American Diabetes Association. 4. Lifestyle management. Diabetes Care. 2017;40(suppl 1):S33-S43.
23. Li Z, Hu Y, Yan R, et al. Twenty minute moderate-intensity post-dinner exercise reduces the postprandial glucose response in Chinese patients with type 2 diabetes. Med Sci Monit. 2018;24:7170-7177.
24. American Diabetes Association. The big picture: checking your blood glucose. www.diabetes.org/diabetes/medication-management/blood-glucose-testing-and-control/checking-your-blood-glucose. Accessed August 23, 2019.
To comment on this article, contact rdavidson@uspharmacist.com.





