US Pharm. 2024;49(1):27-31.
Alzheimer’s disease (AD) presents as one of the most intricate and demanding neurologic conditions encountered in healthcare today. This progressive neurodegenerative disorder is marked by cognitive decline, memory loss, and disruptions in behavior and thinking abilities. As the most prevalent form of dementia, it accounts for roughly 60% to 70% of dementia cases worldwide, gradually progressing from mild memory loss to severe cognitive impairment, affecting daily functioning, and ultimately leading to a loss of independence.1,2
Globally, an estimated 55 million people live with dementia, with AD impacting around 6.7 million older adults in the United States. Projections suggest this number could surge to 13.8 million by 2050. AD stood as the sixth leading cause of death in the U.S. in 2019, ranking fifth among adults aged older than 65 years. The aging population continues to drive the escalating prevalence of AD, posing a substantial healthcare burden.2-5
The ramifications of AD extend far beyond the individuals affected, extending to families, caregivers, and the healthcare system. Caregivers often face considerable emotional and economic challenges, experiencing heightened stress, depression, and financial strain. The costs associated with caring for AD patients, including healthcare, long-term support, and lost productivity, accumulate to billions of dollars annually. Pharmacists play a crucial role in comprehending AD, aiding in its diagnosis, and implementing treatment strategies to enhance the quality of life for both patients and their caregivers. Their involvement is pivotal in addressing the multifaceted challenges posed by this debilitating disease.2
Risk Factors
AD is influenced by a complex interplay of genetic, environmental, and lifestyle factors. While the exact cause of AD remains elusive, advancing age stands as the most prominent risk factor, with its prevalence skyrocketing after age 65 years. Among the elderly population, 5% of individuals aged 65 to 74 years, 13.1% of those aged 75 to 84 years, and a staggering 33.3% of those aged 85 years or older are affected by AD. Women are more susceptible to AD than men, particularly after age 80 years.2,6
Genetics plays a pivotal role in AD pathogenesis. Genetic predispositions, particularly the apolipoprotein E epsilon 4 allele, significantly elevate an individual’s susceptibility to the disease. This allele exerts the strongest influence on late-onset AD, while rare mutations in genes such as the amyloid precursor protein, presenilin 1, and presenilin 2 contribute to familial or early-onset AD. Individuals inheriting these mutations are virtually guaranteed to develop AD if they live a normal life span. Additionally, having a first-degree relative with AD increases an individual’s risk of developing the disease, with the chance further escalating among those with multiple first-degree relatives affected by AD.2,6
While age, genetics, and family history are immutable, several modifiable risk factors can be addressed to reduce the risk of cognitive decline and dementia. By addressing these risk factors, we may be able to prevent or delay up to 40% of dementia cases. Modifiable risk factors for AD encompass a range of lifestyle- and health-related elements. Many factors that increase cardiovascular disease risk, such as diabetes and high cholesterol, are also associated with an increased risk of dementia. Other modifiable risk factors include traumatic brain injury, sleep disturbances, social isolation, and exposure to environmental toxins. Notably, eight modifiable risk factors—midlife obesity, physical inactivity, low education level, depression, smoking, diabetes, hearing loss, and midlife hypertension—have been linked to up to 37% of AD cases. Embracing a healthy lifestyle that incorporates regular physical exercise, a balanced diet rich in fruits, vegetables, and omega-3 fatty acids, and cognitive engagement through lifelong learning and social interactions holds promise in reducing the risk of AD.2,6-9
Clinical Presentation
AD progressively affects memory, cognitive function, and the ability to perform routine tasks. While most diagnosed individuals typically live for 4 to 8 years post diagnosis, some endure the condition for up to 2 decades. The clinical course of AD involves distinct stages: preclinical AD, mild cognitive impairment (MCI) due to AD, and categorized Alzheimer’s dementia with mild, moderate, and severe stages. Symptoms of AD vary across disease stages but encompass diverse manifestations. Initially, individuals may exhibit subtle memory lapses and mild cognitive challenges, often mistaken for typical age-related changes. Memory loss emerges as an early and common sign, leading to difficulties in recalling recent events, conversations, or appointments. Language difficulties arise, causing struggles in finding suitable words or expressing thoughts clearly. As AD progresses, symptoms intensify, with noticeable confusion and disorientation leading to difficulty in recognizing time, location, and familiar faces, occasionally resulting in getting lost in familiar environments. Observable lapses in judgment, such as making impulsive financial decisions or engaging in impaired driving, become more pronounced. Changes in mood and personality, including feelings of depression, anxiety, irritability, or social withdrawal, contribute to the worsening cognitive decline throughout the disease’s course.2,10
Screening and Diagnosis
Screening for and diagnosing AD involve a multifaceted approach encompassing various assessments and tests. Screening often begins with cognitive assessments, which evaluate memory, language, problem-solving, and other cognitive functions through standardized tests like the Mini-Mental State Examination. However, a definitive AD diagnosis requires a more comprehensive evaluation to rule out other potential causes. This includes a thorough medical history review, physical examinations, and laboratory tests. Brain imaging techniques, such as MRI or CT scans, help detect structural brain changes associated with AD, while PET scans with specific tracers can identify abnormal protein accumulation linked to the disease. In some cases, cerebrospinal fluid analysis or genetic testing might be considered for a more accurate diagnosis, especially in cases of early-onset or familial AD. The diagnostic process involves collaboration among healthcare professionals, aiming to distinguish AD from other forms of dementia and pinpointing characteristic patterns of symptoms and biomarkers associated with the disease.6,10,11
Treatment
Currently, there is no cure for AD, but available treatments aim to manage symptoms and slow disease progression. These treatments encompass both pharmacologic and nonpharmacologic interventions, seeking to alleviate symptoms, hinder disease advancement, and improve the overall quality of life for affected individuals.
Previously, treatment options included acetylcholinesterase inhibitors (AChEi) and an N-methyl-D-aspartate receptor antagonist (see TABLE 1). Galantamine and oral rivastigmine, among the cholinesterase inhibitors, are approved for mild-to-moderate AD, while both donepezil and transdermal rivastigmine are approved for all AD stages. There is not a preferred agent within this category, allowing for switching if one proves ineffective or intolerable. Memantine, a receptor antagonist, is recommended for moderate-to-severe AD. AChEi monotherapy is suitable for mild-to-moderate AD, while memantine monotherapy or combination with AChEi is suggested for moderate-to-severe AD. Memantine monotherapy is an alternative for patients who are intolerant to AChEi. Disease progression alone is not a reason to stop AChEi.9,12-17
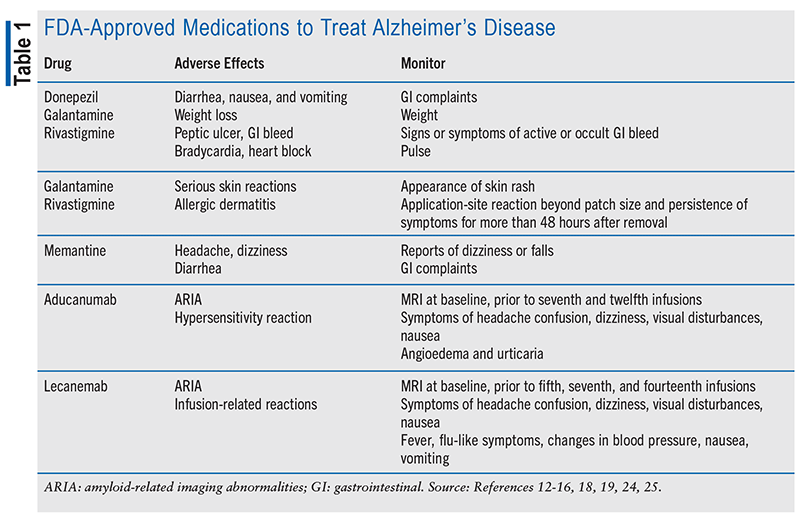
In 2021, the FDA granted accelerated approval to aducanumab, a monoclonal antibody targeting amyloid beta (Aβ) plaques potentially linked to AD. This was the first approval for a new AD treatment in 2 decades. Administered via IV infusion every 4 weeks, aducanumab might slow AD progression by reducing brain Aβ levels. However, common side effects include amyloid-related imaging abnormalities (ARIA), leading to severe consequences like brain swelling or bleeding. Headaches, dizziness, and confusion are also observed. In July 2023, lecanemab, another Aβ-directed monoclonal antibody, obtained FDA approval. Administered via IV infusion every 2 weeks, lecanemab also carries risks of ARIA and infusion reactions, characterized by fever, chills, headache, rash, nausea, vomiting, abdominal discomfort, and increased blood pressure; prophylactic management with anti-inflammatory therapies and diphenhydramine may be required. Both drugs are recommended for individuals with MCI or mild dementia and are not advised for advanced dementia stages. No safety or efficacy data exist for earlier or later stages of disease progression. Confirmation of Aβ pathology through MRI before treatment and subsequent MRIs during infusions for both drugs are required.18,19
Nonpharmacologic treatments for AD encompass a diverse range of interventions aimed at improving quality of life, managing symptoms, and supporting overall well-being in individuals affected by the condition. These interventions often focus on enhancing cognitive function, promoting social engagement, and maintaining functional abilities. Nonpharmacologic strategies include cognitive stimulation programs, such as memory training and structured activities designed to engage and challenge cognitive abilities. Behavioral interventions aim to manage behavioral and psychological symptoms through techniques such as behavior modification and tailored communication approaches. Additionally, psychosocial interventions involve support groups for both patients and caregivers, offering emotional support and education on coping strategies. Physical exercise, diet modification, and music therapy have also shown promise in improving cognitive function and overall health in individuals with AD. The multifaceted nature of nonpharmacologic treatments emphasizes their role in complementing pharmacologic approaches, enhancing the quality of life, and supporting individuals affected by AD and their caregivers.20,21
Role of the Pharmacist
Pharmacists play a crucial role in managing AD across various facets of care. They serve as invaluable sources of knowledge, educating patients, caregivers, and healthcare professionals about available treatments, potential side effects, and methods for medication adherence. Trained to recognize medications such as anticholinergics, benzodiazepines, proton pump inhibitors, and specific pain medications, as well as chronic conditions that heighten the risk of AD, pharmacists review patient records to foresee potential risks even before symptoms emerge. Upon identifying risks, pharmacists provide guidance to patients regarding the connection between their medications and AD, discussing lifestyle adjustments such as dietary changes, exercise, and cognitive activities to potentially reduce these risks. Working closely with healthcare teams, pharmacists fine-tune medication plans, ensuring correct dosages, monitoring for interactions, and addressing medication-related concerns for AD patients. They navigate the intricate landscape of medication management throughout the disease’s progression, adapting treatments to suit changing needs while enhancing understanding for patients and caregivers, significantly contributing to improving the quality of life for those affected by AD. Beyond their clinical responsibilities, pharmacists offer attentive support, assisting patients and caregivers in managing the emotional complexities of the disease, facilitating connections with additional resources and support systems to foster community and alleviate feelings of isolation.22,23
Conclusion
AD represents a complex and challenging neurologic condition with profound impacts on individuals, families, and healthcare systems. As the most common form of dementia, it progressively steals memories, disrupts lives, and burdens economies. An aging population fuels its rise, with escalating mortality and financial costs echoing the disease’s devastating impact. Beyond the individual, caregivers and healthcare systems grapple with emotional strain and financial hardship. Pharmacists assume a pivotal role in AD management, serving as educators, counselors, and medication experts. They recognize medications and health conditions that raise the risk, empowering patients with lifestyle changes to fight back. Pharmacists collaborate with healthcare teams to optimize medication regimens, monitor for interactions, and address concerns, significantly enhancing patient understanding and well-being. Beyond clinical support, pharmacists provide emotional assistance, connecting patients and caregivers with vital resources and support networks, fostering a sense of community and resilience. Their multifaceted role underscores their immense contribution to AD care, offering guidance, compassion, and comprehensive support to those navigating the complexities of this debilitating disease.

What Is Alzheimer’s Disease?
Alzheimer’s disease (AD) is a progressive brain disease that slowly destroys memory, thinking, and eventually the ability to carry out simple tasks. It is the most common form of dementia and impacts daily life as it worsens over time.
What Causes AD?
The exact cause is unknown, but it is believed to involve abnormal buildup of protein fragments in the brain called amyloid plaques and tau tangles. These disrupt brain cell communication and function, leading to memory loss and cognitive decline.
What Are the Symptoms of AD?
AD starts slowly and can be hard to spot at first. In the early stages, you might notice small things, like forgetting names or misplacing items. You might see that it is tough to remember recent events or information. This can lead to repeating stories or asking for things to be said again so that you can remember them. If someone tells you, “I already told you that,” or if you find yourself saying the same thing more than once, it could be a sign of Alzheimer’s. Other things to watch for are problems with talking, trouble concentrating or figuring things out, finding it hard to do tasks like paying bills or cooking, and getting lost in places you know well.
Am I at Risk for AD?
Age is the biggest risk factor for AD. For those aged older than 65 years, the risk increases significantly. Genetics also plays a role, with family history a strong indicator. Other risk factors include head injuries, heart disease, high blood pressure, diabetes, depression, lack of physical activity, and smoking.
Is There a Cure?
Currently, no cure for AD exists; however, medications can help manage symptoms and slow progression. Nonpharmacologic interventions such as cognitive stimulation, exercise, and social engagement can also be beneficial.
Can I Prevent AD?
See your doctor regularly; early diagnosis is crucial for managing symptoms and accessing support. You should also stay active and eat a healthy diet rich in fruits, vegetables, and whole grains. You also want to ensure that you connect with others and stay socially active.
Where Can I Go for More Information?
It is always important for you to speak with your pharmacist or healthcare provider when you have questions regarding AD. Some websites you can refer to include:
Alzheimer’s Association: www.alz.org
Alzheimer’s Disease International: www.alzint.org
National Institute on Aging: www.alzheimers.gov
CDC Healthy Aging Program: www.cdc.gov/aging
REFERENCES
1. CDC. Alzheimer’s disease and related dementias. CDC. October 26, 2020. www.cdc.gov/aging/aginginfo/alzheimers.htm. Accessed December 2, 2023.2. 2023 Alzheimer’s disease facts and figures. Alzheimers Dement. 2023;19(4):1598-1695.
3. WHO. Dementia. March 15, 2023. www.who.int/news-room/fact-sheets/detail/dementia. Accessed December 2, 2023.
4. Hebert LE, Weuve J, Scherr PA, Evans DA. Alzheimer disease in the United States (2010–2050) estimated using the 2010 census. Neurology. 2013;80(19):1778-1783.
5. CDC. Mortality in the United States, 2019. December 22, 2020. www.cdc.gov/nchs/products/databriefs/db395.htm. Accessed December 2, 2023.
6. Scheltens P, De Strooper B, Kivipelto M, et al. Alzheimer’s disease. Lancet. 2021;397(10284):1577-1590.
7. Nianogo RA, Rosenwohl-Mack A, Yaffe K, et al. Risk factors associated with Alzheimer disease and related dementias by sex and race and ethnicity in the US. JAMA Neurol. 2022;79(6):584-591.
8. Edwards III GA, Gamez N, Escobedo Jr. G, et al. Modifiable risk factors for Alzheimer’s disease. Front Aging Neurosci. 2019;11:146.
9. Livingston G, Huntley J, Sommerlad A, et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet. 2020;396(10248):413-446.
10. Knopman DS, Amieva H, Petersen RC, et al. Alzheimer disease. Nat Rev Dis Primer. 2021;7(1):33.
11. Alzheimer’s Association. Medical tests for diagnosing Alzheimer’s. www.alz.org/alzheimers-dementia/diagnosis/medical_tests. Accessed December 2, 2023.
12. National Library of Medicine. DONEPEZIL-donepezil hydrochloride tablet. DailyMed. www.dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=11ac01f4-d26e-47b2-9660-d514ab097fdb. Accessed December 2, 2023.
13. National Library of Medicine. EXELON-rivastigmine patch, extended release. DailyMed. www.dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=07c12e14-bbd5-4ec8-84e4-fbe7b5ba10e0. Accessed December 2, 2023.
14. National Library of Medicine. EXELON-rivastigmine tartrate capsule. DailyMed. https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=fead084a-a376-4d57-bdf0-aa7ed3655505. Accessed December 2, 2023.
15. National Library of Medicine. RAZADYNE-galantamine hydrobromide tablet, film coated. DailyMed. www.dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=e62efb5a-d2cc-4e11-9e61-10e65ef3d897. Accessed December 2, 2023.
16. National Library of Medicine. NAMENDA-memantine hydrochloride tablet. DailyMed. www.dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=bc6e059d-1951-4fa3-b904-230d4165724d. Accessed December 2, 2023.
17. National Institute for Health and Care Excellence. Dementia: assessment, management and support for people living with dementia and their carers. June 20, 2018. www.nice.org.uk/guidance/ng97. Accessed December 2, 2023.
18. Cummings J, Aisen P, Apostolova LG, et al. Aducanumab: appropriate use recommendations. J Prev Alzheimers Dis. 2021;8(4):398-410.
19. Cummings J, Apostolova L, Rabinovici GD, et al. Lecanemab: appropriate use recommendations. J Prev Alzheimers Dis. 2023;10(3):362-377.
20. Cho E, Shin J, Seok JW, et al. The effectiveness of non-pharmacological interventions using information and communication technologies for behavioral and psychological symptoms of dementia: a systematic review and meta-analysis. Int J Nurs Stud. 2023;138:104392.
21. Berg-Weger M, Stewart DB. Non-pharmacologic interventions for persons with dementia. Mo Med. 2017;114(2):116-119.
22. Riachi M. How pharmacists can help their dementia patients. Can Pharm. 2016;149(2):67-69.
23. Collins S. From the community to the clinic, pharmacists have a critical role to play in dementia care. American Pharmacists Association. www.pharmacist.com/Publications/Pharmacy-Today/Article/from-the-community-to-the-clinic-pharmacists-have-a-critical-role-to-play-in-dementia-care. Accessed December 4, 2023.
24. National Library of Medicine. ADUHELM-aducanumab injection, solution. DailyMed. www.dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=41706573-546f-6774-6872-5374726f6e67. Accessed December 2, 2023.
25. National Library of Medicine. LEQEMBI-lecanemab injection, solution. DailyMed. www.dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=9d1ff786-e577-410a-a273-c4d7d0e4e975. Accessed December 2, 2023.
The content contained in this article is for informational purposes only. The content is not intended to be a substitute for professional advice. Reliance on any information provided in this article is solely at your own risk.
To comment on this article, contact rdavidson@uspharmacist.com.





