US Pharm. 2024;49(7):29-38.
ABSTRACT: Tuberculosis (TB) is a widespread contagious bacterial infection that is transmitted through the inhalation of airborne droplets. Early detection and treatment of TB are crucial for preventing disease progression, reducing transmission, and improving treatment outcomes. Factors to consider when developing an appropriate treatment plan for the patient depend on the type and severity of TB diagnosed, the presence of drug resistance, and patient characteristics. In collaboration with healthcare providers, pharmacists can play a key role in creating an appropriate TB drug regimen for each patient. Ensuring medication adherence while monitoring for drug interactions or adverse reactions is integral to enhancing patient safety and treatment efficacy in TB management.
Tuberculosis (TB) is a contagious airborne bacterial infection that afflicts a significant portion of the world’s population. This disease starts primarily in the lungs, but it can spread to other parts of the body. When TB resides in the body without producing any symptoms of the disease, the affected individual is an asymptomatic carrier.1
Pathophysiology
TB is caused by the Mycobacterium tuberculosis pathogen. When a person with active TB sneezes, coughs, or speaks, aerosol droplets containing M tuberculosis may be transferred to people nearby if they inhale the droplets. Upon entering the new host, the TB bacteria spread to the respiratory system and travel into the lungs. The host’s immune system then activates to fight the infection, and the bacteria are engulfed by alveolar macrophages. If the macrophages are unable to eradicate the bacilli from the system, the bacteria proliferate and release within the intracellular environment, where they are engulfed by other alveolar macrophages. The cycle then repeats, and lymphocytes travel to the site of infection and initiate a cell-mediated immune response to attack and prevent further replication of the bacteria.2 The host typically exhibits no signs or symptoms during this stage, and the bacteria potentially are eliminated or enter a latent stage within the granuloma.1,2 The granuloma’s center may undergo necrosis caused by the breakdown of the host’s immune cells, leading to a caseous granuloma. Foamy macrophages form and spread around the necrotic foci of the granuloma. M tuberculosis also causes a dysregulation in lipid metabolism that helps form the foam cells that accumulate in the caseum of the granuloma.
Subsequently, during late-stage TB, the cell-wall core softens, leading to active TB and the transmission of infectious bacteria to a new host. When the host’s immune system is compromised, the inactive bacilli in the granuloma can reactivate and multiply; this causes the granuloma to soften and liquefy, resulting in the formation of cavities in the granuloma.2,3 The granuloma’s structure then weakens, releasing the bacteria.
Risk Factors
Persons at risk for contracting TB include those who are in close contact with other people who are already infected. Individuals from countries with high TB rates, such as India, Pakistan, the Philippines, Nigeria, and China, are at risk for contracting the infection.2,4 Also at high risk are people who work or live in homeless shelters, nursing homes, and hospitals. Patients who have a compromised immune system, such as those with HIV/AIDS, diabetes, severe kidney disease, and organ transplants, have a greater risk of contracting TB. Young children and infants are also more susceptible to becoming infected.
Signs and Symptoms
Symptoms and signs of active TB often depend on the location of bacterial growth within the body. For pulmonary TB specifically, signs and symptoms may include chest pain, a bad cough lasting 3 weeks or longer, and coughing up blood or sputum.3 Other symptoms and signs of TB are weakness, fatigue, loss of appetite, chills, fever, and night sweats.
Screening
There are two methods of testing for the presence of TB bacteria in the body: the tuberculin skin test (TST; e.g., Mantoux test) and the TB blood test (e.g., interferon-gamma [IFN-gamma] release assay [IGRA]). These tests determine whether a patient has been infected with the bacteria, but they do not indicate whether the patient’s TB infection is latent or has progressed to active disease.3 The Mantoux test (also known as the purified protein derivative [PPD] test), which uses a dose of PPD injected intradermally, is most frequently performed to assess TB exposure. In low-risk patients with minimal TB exposure, the Mantoux test is considered positive if the induration measures approximately 15 mm. For intermediate-risk patients, an induration >10 mm indicates a positive result, and patients who have a high risk of exposure to TB are considered positive if the induration is >5 mm.1
IGRAs have similar sensitivity to the Mantoux test, but they are more specific. TB blood tests measure the presence of inflammatory cytokines, particularly IFN-gamma. The benefit of performing a test involving antigen-specific stimulation of IFN-gamma release is that a single blood sample is required, eliminating the need for multiple visits to interpret the results. The blood sample may also be utilized for HIV screening and other testing. The cost and the need for technical expertise are drawbacks to the use of IGRAs such as QuantiFERON.1
Diagnosis
TB should always be suspected in patients who present with signs and symptoms of TB, and patients should undergo a comprehensive medical evaluation. A patient is diagnosed with latent TB infection if the screening result is positive but the medical evaluation shows no indication of TB. The diagnostic process begins with an assessment of the patient’s medical history and a thorough physical examination, followed by a chest x-ray and additional laboratory tests.5,6
The clinician should ask the patient for details regarding the TB exposure or disease while also considering demographic factors that can increase the risk of TB exposure. It is also important to take into account medical conditions such as HIV or diabetes, which can increase the likelihood of latent TB infection progressing to active TB. The physical examination, in addition to delineating the patient’s overall condition, is used to help determine an appropriate treatment plan for potential TB. Screening is then performed to detect the presence of TB infection. If the test is positive, a chest x-ray is obtained to assess for chest abnormalities.5 Lesions of varying sizes and shapes can occur anywhere in the lungs and and may suggest the possibility of pulmonary TB. A sputum smear may also be taken to check for the presence of acid-fast bacilli (AFB), often an indicator of TB. This test is quick and easy to perform; however, it does not confirm the TB diagnosis because not all AFB are M tuberculosis. The patient also must be tested early for the presence of drug resistance to ensure that the appropriate treatment regimen is implemented.1,3
Treatment
TB is treated with antibiotics such as isoniazid (INH), rifampin (RIF), pyrazinamide (PZA), streptomycin, and ethambutol (EMB).4 Treatment can last for 3, 4, 6, or 9 months, depending on the regimen. Treatment aims to cure the patient as well as lower the likelihood of transmitting the disease to others.
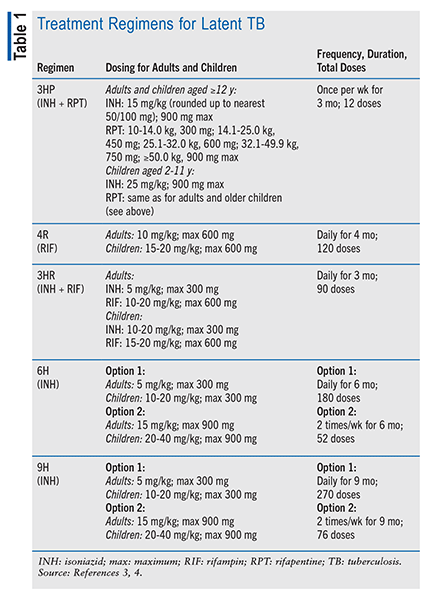
Latent TB Treatment: Many different factors can trigger the transformation of latent TB into an active infection. Malnutrition, chronic renal failure, drug or alcohol addiction, uncontrolled diabetes, and smoking are some of the potential causes of TB reactivation. Antibiotics including INH, rifapentine (RPT), and RIF, administered singly or in combination, are used to treat latent TB infection.6 The CDC recommends a short course of treatment (3 or 4 months) for latent TB infections (TABLE 1) based on greater efficacy and safety. The recommended short-course regimens include INH plus RPT once weekly for 3 months (3HP), RIF daily for 4 months (4R), and INH plus RIF daily for 3 months (3HR).2,3 INH monotherapy lasting 6 months (6H) or 9 months (9H) is an alternative if the short-course regimens are not available or are not a viable option. INH monotherapy regimens are effective, but because of the treatment length they carry a greater toxicity risk and lower treatment-completion rates. The INH monotherapy regimen should be stopped if the patient experiences loss of appetite, nausea or vomiting, or rash; other adverse drug reactions are yellowing of the skin or eyes and cola-colored urine or light-colored stools. Patients taking RIF or RPT should be advised that the color of their urine or other bodily fluids may turn orange.3
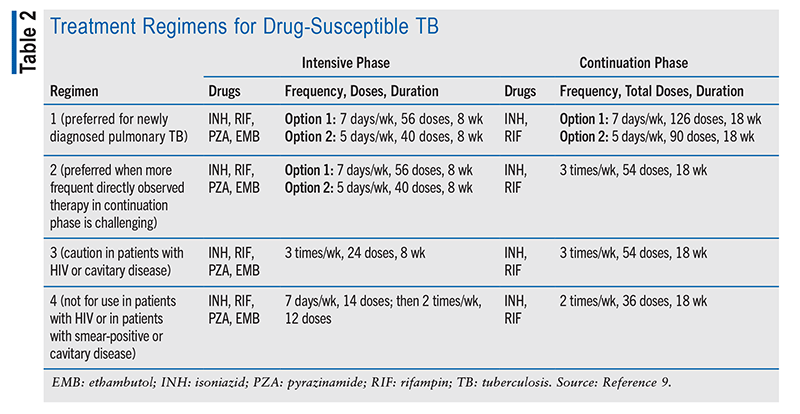
Drug-Susceptible TB Treatment: If the disease is active and not suspected to be drug-resistant, the treatment regimen recommended for adults by the American Thoracic Society/CDC/Infectious Diseases Society of America (IDSA) clinical practice guidelines involves a 2-month phase of INH, RIF, PZA, and EMB followed by INH and RIF continued for a 4-month phase (TABLE 2).7-9 When drug-susceptibility test (DST) results show that the patient is susceptible to INH and RIF, EMB may be discontinued while continuing the INH, RIF, and PZA.10,11 Side effects are similar to the side effects for latent-TB treatment regimens, including loss of appetite, nausea, vomiting, brown urine, and jaundice.9 While on this regimen, patients may experience blurred vision, which should be reported immediately.
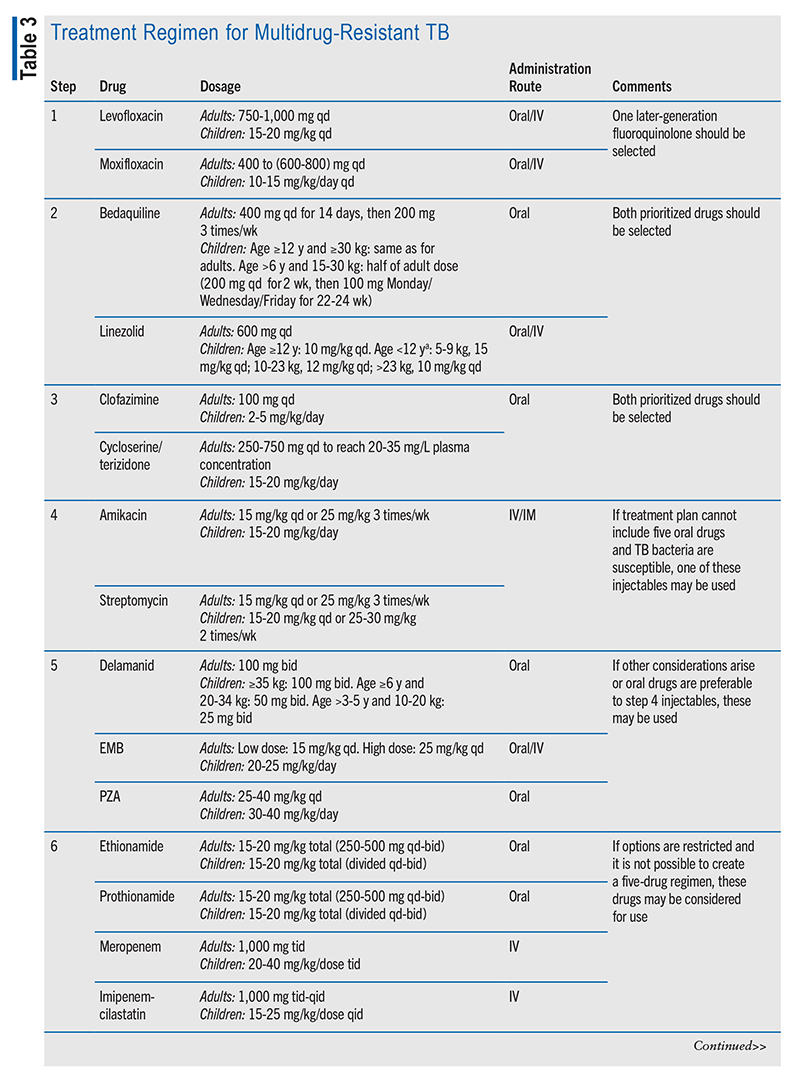
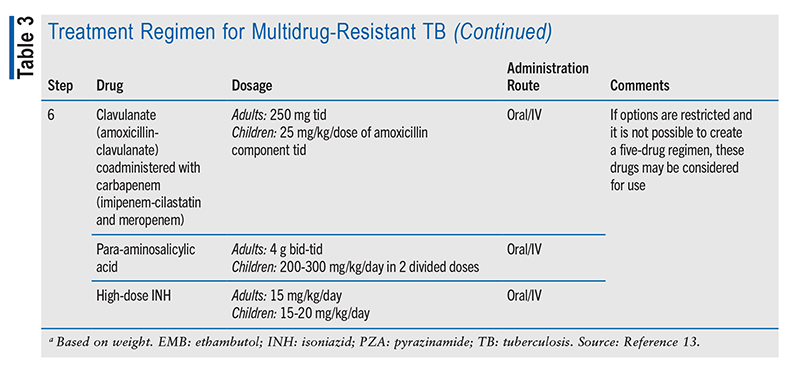
Multidrug-Resistant TB (MDR-TB) Treatment: MDR-TB involves resistance to at least two first-line antibiotic treatments (INH and RIF). Patients who do not complete or are nonadherent to the treatment regimen can develop MDR-TB.12,13 MDR-TB treatment typically uses second-line therapies and is more expensive. When MDR-TB is suspected or confirmed, a consultation must be scheduled with a TB specialist. A molecular DST should be performed to detect any mutation associated with resistance.13-15 If any resistance to RIF is detected, another DST should be performed for other first-line drugs, aminoglycosides, and fluoroquinolones. Any drugs found ineffective because of bacterial resistance should not be included in the treatment regimen.16 The IDSA recommends that the initial/intensive phase of treatment include at least five effective drugs.13 This guidance is for treatment that is personalized to the DST findings. A six-step regimen is created using five or more agents to which the bacteria are susceptible (TABLE 3).
The CDC recommends that MDR-TB be treated with a regimen that contains pretomanid, bedaquiline, and linezolid (BPaL). Pretomanid may be continued for up to 9 months in the BPaL regimen if the patient has a delayed response to treatment during the first 8 weeks. Pretomanid is approved for the treatment of pulmonary TB, but it is not approved for use as monotherapy. According to the CDC, the BPaL regimen should comprise pretomanid 200 mg orally daily for 26 weeks; bedaquiline 400 mg orally every day for 2 weeks, followed by 200 mg orally three times per week with ≥48 hours between doses; and linezolid 600 mg orally every day. If adverse events occur with linezolid, either the dosage should be decreased to 300 mg daily or a dosing interruption should be implemented.13,14
The FDA advises that the use of fluoroquinolone antibacterial drugs should be monitored because of the potential serious adverse effects. These effects, which involve the tendons, joints, nerves, muscles, and central nervous system, can potentially become permanent.15 Patients should stop taking fluoroquinolones if they experience tendon, joint, and muscle pain; hallucination; or confusion.17
The Pharmacist’s Role
Pharmacists play an integral part in the treatment of TB from initial diagnosis to completion of therapy. To ensure safe and effective treatment, pharmacists can collaborate with other healthcare providers to determine the optimal TB medication therapy for each patient. Drug-susceptibility testing, patient risk factors, and treatment guidelines must be taken into consideration when selecting the appropriate therapy.18,19 TB treatment can be long and tedious, often lasting several months, which can pose challenges for medication adherence.20 Understanding these challenges equips pharmacists to provide effective patient counseling and support and enables them to address barriers to adherence and create strategies for helping patients maintain a consistent treatment regimen. Throughout treatment, pharmacists can educate and monitor patients on the potential side effects and drug interactions that can occur while taking these drugs. Overall, pharmacists have a key role in fostering the success of TB treatment through their knowledge of medication management and involvement in patient education.
Conclusion
TB remains a major health challenge that causes serious harm to individuals and communities worldwide. All forms of TB (latent, drug-susceptible, and MDR) require complex treatment regimens. The objective of TB management is to treat and cure the patient’s infection while also preventing the disease from spreading to others. The proper management of TB also helps minimize the occurrence of new drug-resistant strains. Other treatment-related goals include managing the patient’s symptoms and improving the patient’s quality of life. The collaboration of pharmacists and other healthcare providers in the comprehensive management of TB helps ensure treatment safety and efficacy while also achieving treatment success, monitoring drug resistance, and contributing to the global effort to fight this widespread contagious disease.
REFERENCES
1. Adigun R, Singh R. Tuberculosis. In: StatPearls [Internet]. Treasure Island, FL: StatPearls Publishing; 2023-.
2. Alsayed SSR, Gunosewoyo H. Tuberculosis: pathogenesis, current treatment regimens and new drug targets. Int J Mol Sci. 2023;24(6):5202.
3. CDC. Tuberculosis (TB). www.cdc.gov/tb/index.html. Accessed June 11, 2024.
4. Lienhardt C. From exposure to disease: the role of environmental factors in susceptibility to and development of tuberculosis. Epidemiol Rev. 2001;23(2):288-301.
5. American Lung Association. Treating and managing tuberculosis. www.lung.org/lung-health-diseases/lung-disease-lookup/tuberculosis/treating-and-managing. Accessed April 11, 2024.
6. Peloquin CA, Davies GR. The treatment of tuberculosis. Clin Pharmacol Ther. 2021;110(6):1455-1466.
7. National Library of Medicine. Pyrazinamide tablet. In: DailyMed [database]. https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=ecee3128-a47f-4ced-a1db-4f58c5f3e9c6. Accessed April 11, 2024.
8. National Library of Medicine. Ethambutol hydrochloride tablet. In: DailyMed [database]. https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=2f857930-d002-4abe-9907-bfd8fd7fe5ed. Accessed April 11, 2024.
9. Nahid P, Dorman SE, Alipanah N, et al. Official American Thoracic Society/Centers for Disease Control and Prevention/Infectious Diseases Society of America clinical practice guidelines: treatment of drug-susceptible tuberculosis. Clin Infect Dis. 2016;63(7):e147-e195.
10. Rifadin (rifampin capsules) and Rifadin IV (rifampin IV) product information. Bridgewater, NJ: sanofi-aventis U.S. LLC; November 2010.
11. Isoniazid (tablets) product information. Princeton, NJ: Sandoz Inc; July 2016.
12. American Lung Association. Tuberculosis symptoms and diagnosis. www.lung.org/lung-health-diseases/lung-disease-lookup/tuberculosis/symptoms-diagnosis. Accessed April 11, 2024.
13. Nahid P, Mase SR, Migliori GB, et al. Treatment of drug-resistant tuberculosis. An official ATS/CDC/ERS/IDSA clinical practice guideline. Am J Respir Crit Care Med. 2019;200(10):e93-e142.
14. CDC. Provisional CDC guidance for the use of pretomanid as part of a regimen [bedaquiline, pretomanid, and linezolid (BPaL)] to treat drug-resistant tuberculosis disease. www.cdc.gov/tb/hcp/treatment/bpal.html. Accessed June 6, 2024.
15. National Institute of Allergy and Infectious Diseases. Tuberculosis drugs and mechanisms of action. www.niaid.nih.gov/diseases-conditions/tbdrugs. Accessed April 11, 2024.
16. Early Childhood Learning and Knowledge Center. Tuberculosis. September 25, 2023. https://eclkc.ohs.acf.hhs.gov/physical-health/article/tuberculosis. Accessed April 11, 2024.
17. FDA. FDA drug safety communication: FDA advises restricting fluoroquinolone antibiotic use for certain uncomplicated infections; warns about disabling side effects that can occur together. www.fda.gov/drugs/drug-safety-and-availability/fda-drug-safety-communication-fda-advises-restricting-fluoroquinolone-antibiotic-use-certain. Accessed May 1, 2024.
18. CDC. Travelers’ health: tuberculosis (TB). wwwnc.cdc.gov/travel/diseases/tuberculosis. Accessed April 11, 2024.
19. American Lung Association. Learn about tuberculosis. www.lung.org/lung-health-diseases/lung-disease-lookup/tuberculosis/learn-about-tuberculosis. Accessed April 11, 2024.
20. Johns Hopkins Medicine. Tuberculosis (TB). www.hopkinsmedicine.org/health/conditions-and-diseases/tuberculosis-tb. Accessed April 11, 2024.
The content contained in this article is for informational purposes only. The content is not intended to be a substitute for professional advice. Reliance on any information provided in this article is solely at your own risk.
To comment on this article, contact rdavidson@uspharmacist.com.





