US Pharm. 2024;49(6):35-40.
ABSTRACT: As healthcare costs and the number of individuals diagnosed with and treated for medical conditions continue to rise, the availability of generic drugs gives patients increased access to affordable, safe, and effective therapies and can enhance medication adherence. FDA approval of first-time generic drugs is an important and beneficial component of healthcare, as these approvals mark the first generic version of a specific brand-name drug, providing patients with more affordable options for treatment. The approval of generic medications can also strengthen the drug-supply chain while reducing the risk of drug shortages for certain products. Pharmacists can be instrumental in educating patients about the availability of first-time generic drug approvals and reassuring them of these products’ safety, efficacy, and equivalence to the brand-name counterpart.
Access to affordable medications remains a top priority for patients nationwide. As healthcare costs and the number of individuals diagnosed and treated for various medical conditions continue to increase, the availability of generic drugs provides patients with expanded access to affordable, safe, and effective therapies. Before brand-name and generic drugs are approved, the FDA rigorously evaluates them using scientific and regulatory measures to ensure their safety, efficacy, and quality. The FDA requires all manufacturers of generic drugs to adhere to and meet the equivalent batch-to-batch strength, purity, and quality requirements as their brand-name drugs and to observe the same strict Current Good Manufacturing Practice (CGMP) regulations.1 CGMP standards guarantee proper design, monitoring, and regulation of manufacturing processes and facilities.1 The FDA estimates that 91% of all prescriptions dispensed in the United States are for generics, and to date more than 32,000 generic drugs have been approved.2,3 The Association of Accessible Medicines reports that although generic drugs comprise a sizable percentage of prescriptions dispensed in the U.S., they account for only 17.5% of total drug costs.4
Generic drugs give prescribers and patients expanded treatment options that are safe and effective as well as generally less expensive than their brand-name counterparts. According to the FDA, the growing availability of generic drugs ultimately benefits patients because the increased marketplace competition lowers the cost of treatment and renders healthcare more accessible to a greater number of patients.2,3
Overview
First-time generic drugs are reported to account for an estimated 10% of all generics approved annually, and the FDA has approved more than 450 first-time generic drugs since 2015.5 The FDA defines a first-time generic application as any received first-to-file Abbreviated New Drug Application (ANDA) that is eligible for 180-day exclusivity or has no blocking patents or exclusivities and for which no ANDA for the drug product was previously approved.6
Approval Process for Generics
The FDA’s Center for Drug Evaluation and Research (CDER) approves a broad array of new drugs annually, including safe, effective, and high-quality generic options that are typically less expensive than their brand-name products.6 A sizable number of first-time generic drugs are approved each year; for example, in 2022 the FDA approved 107 such drugs, and in 2023 it approved 90.7 For some drugs, multiple manufacturers have applied for approval of a generic.
The FDA requires manufacturing, packaging, and evaluation sites for generic drugs to pass quality standards equivalent to those for the brand-name drugs; additionally, the generic drug must undergo a thorough evaluation and review process.2,8 The FDA’s Office of Generic Drugs (OGD) supervises the regulatory process to ensure the safety and efficacy of drugs being considered for approval; determines and implements science initiatives to research generic drugs; publishes clinical data and reports on generic drug development and review; and presents educational materials and information for prescribers, other healthcare providers, and patients.2,8
Recent First-Time Generic Drug Approvals
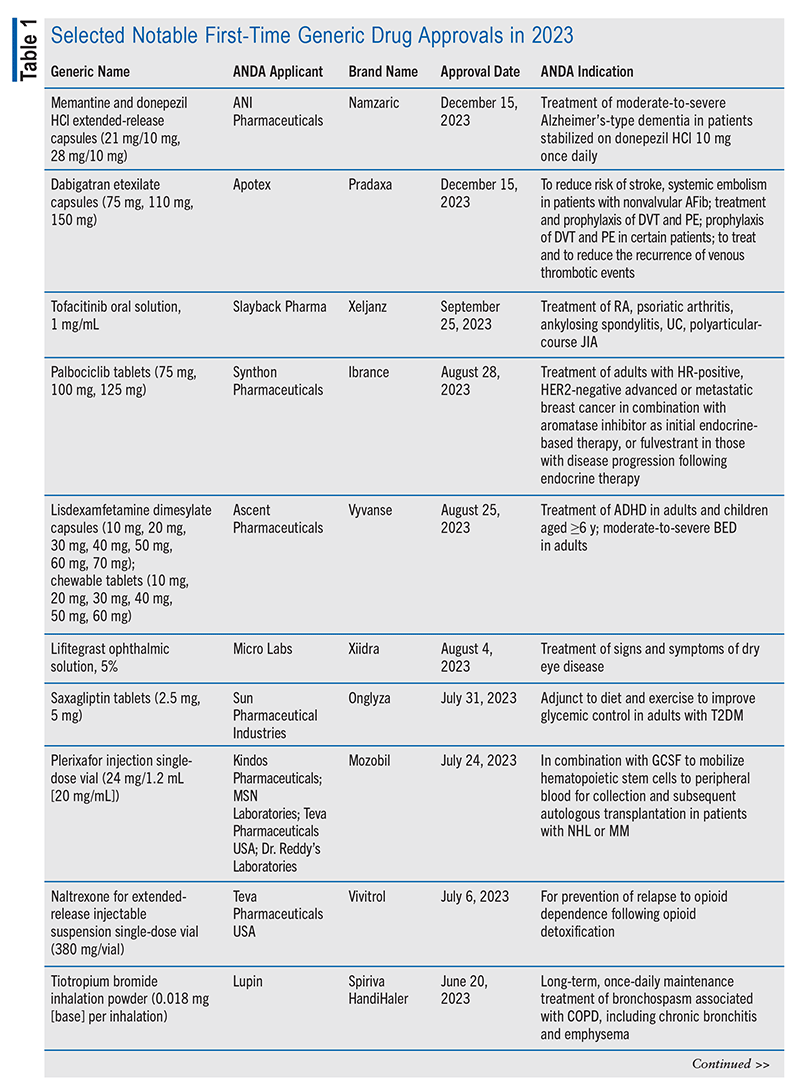
In its 2023 annual report, the OGD stated that one of its notable generic approvals that year was the first generic naltrexone extended-release injectable suspension (for the brand-name product Vivitrol), which is of prime importance given the ongoing opioid epidemic.9 The OGD added, “Critical to the approval of this product were research collaborations to establish methods to assess its bioequivalence which uses complex, long-acting, injectable, biodegradable, polymer-microsphere technology.”9 A selection of 2023 approvals deemed significant by the OGD are summarized in TABLE 1.9

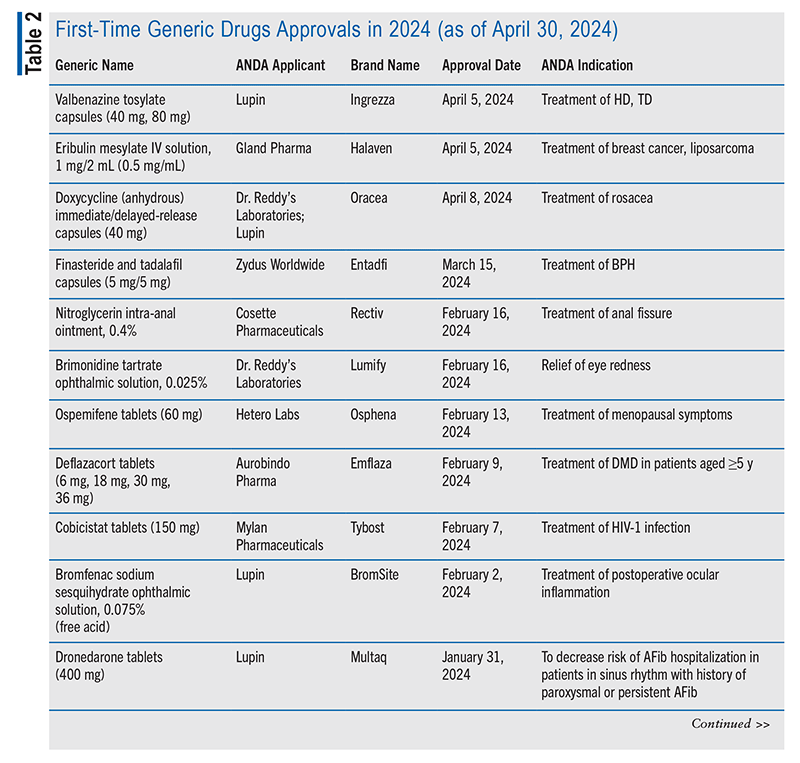
FDA first-time generic drug approvals for 2024 (up to April 30, 2024) are summarized in TABLE 2.6,10 Comprehensive lists of first-time generic drug approvals from previous years are available on the FDA’s First Generic Drug Approvals website.7

Current Research Findings
FDA researchers performed a cross-sectional study to analyze the marketing trajectory of 687 first-time generic drug products approved between January 1, 2010, and June 30, 2017.11 The resulting report was published by the FDA in 2021. The researchers determined that FDA-approved first-time generic drugs result in potential savings for both patients and the healthcare system; however, for the savings to be maximized, the generic product must be marketed in a timely manner following its approval.11
According to the OGD’s 2023 annual report, the approval of generic drugs from multiple manufacturers can strengthen the drug-supply chain while also reducing the risk of drug shortages for some drug products.9,12 The report added that first-time generics provide patients access to affordable medications in cases where no generic competition previously existed. It also was noted that the cost savings resulting from the approval and availability of generic medications amounted to billions of dollars in the past decade.9,12
In a recent CDER publication, OGD director Iilun Murphy, MD, discussed educational initiatives and research efforts that the OGD has implemented to expand awareness about generics and to gain better insight into prescriber and patient perceptions about generic drugs.13 Dr. Murphy noted that increased educational efforts could help dispel common myths and misconceptions about the safety, quality, and effectiveness of generic drugs and lead to more patients being likely to use them, which has the potential to improve patient access and adherence as well as overall clinical outcomes.13
The Pharmacist’s Role
The instrumental and multifaceted role of pharmacists across practice settings is well documented. Pharmacists’ drug expertise and frequent patient interactions put them in a key position to positively impact patient adherence and overall clinical outcomes as well as make patient-centered clinical recommendations for preventing, treating, and managing a broad range of medical conditions. During counseling, the pharmacist can educate patients about generic versus brand-name drugs and reassure them that the FDA evaluates generic medications to make sure that they are as safe and effective as their brand-name counterparts. The pharmacist can also explain to patients that the FDA ensures that every generic drug contains the identical active ingredient and meets the same high standards as the brand-name drug; has inactive ingredients shown to be safe; possesses equal strength, stability, quality, and performance characteristics; has equivalent safety and efficacy and pharmacologic effects, indications, and clinical benefits; and comes in the same dosage form and administration route as the brand-name product.12-14 The pharmacist can also let patients know about approvals of first-time generic drugs, thereby expanding their access to medications and saving them money.
Conclusion
Generic drugs are associated with significant cost savings as well as improved medication adherence because they increase patients’ access to safe and effective drug products that are equivalent to the brand-name counterpart. As clinicians and patient educators, pharmacists can increase awareness about the clinical benefits of generic drugs and keep patients informed about ongoing approvals of first-time generic drugs that can save them money and enhance treatment continuity and adherence.
REFERENCES
1. FDA. Facts about the Current Good Manufacturing Practice (CGMP). www.fda.gov/drugs/pharmaceutical-quality-resources/facts-about-current-good-manufacturing-practice-cgmp. Accessed March 22, 2024.
2. FDA. Generic drugs. www.fda.gov/drugs/buying-using-medicine-safely/generic-drugs. Accessed March 25, 2024.
3. FDA. Office of Generic Drugs 2022 annual report. www.fda.gov/drugs/generic-drugs/office-generic-drugs-2022-annual-report. Accessed March 25, 2024.
4. Association for Accessible Medicines. Generic medicines. https://accessiblemeds.org/generic-medicines. Accessed March 25, 2024.
5. Turner S. Pathbreakers: the journey of first generics. Pharmaceutical Technology. www.pharmaceutical-technology.com/features/pathbreakers-the-journey-of-first-generics. Accessed March 25, 2024.
6. FDA. First-time generic drug approvals 2024. www.fda.gov/drugs/drug-and-biologic-approval-and-ind-activity-reports/first-generic-drug-approvals. Accessed March 25, 2024.
7. FDA. ANDA (generic) drug approvals—previous years. www.fda.gov/drugs/first-generic-drug-approvals/anda-generic-drug-approvals-previous-years. Accessed March 25, 2024.
8. FDA. Generic drug facts. www.fda.gov/drugs/generic-drugs/generic-drug-facts. Accessed March 26, 2024.
9. FDA. Office of Generic Drugs 2023 annual report. www.fda.gov/drugs/generic-drugs/office-generic-drugs-2023-annual-report. Accessed March 25, 2024.
10. Drugs.com. Latest generic drug approvals: first-time generic approvals 2024. www.drugs.com/generic-approvals.html. Accessed April 30, 2024.
11. Chahal HS, Patel R, Shimer M. Research Report: Marketing of First Generic Drugs Approved by U.S. FDA From January 2010 to June 2017. Silver Spring, MD: U.S. Food & Drug Administration; 2021.
12. FDA. Office of Generic Drugs. www.fda.gov/about-fda/center-drug-evaluation-and-research-cder/office-generic-drugs. Accessed March 25, 2024.
13. FDA. Improving medication adherence and patient experience by researching patient perceptions of generic drugs. www.fda.gov/drugs/cder-conversations/improving-medication-adherence-and-patient-experience-researching-patient-perceptions-generic-drugs. Accessed March 26, 2024.
14. FDA. FDA fact sheet: what’s involved in reviewing and approving generic drug applications? www.fda.gov/media/99163/download. Accessed March 26, 2024.
The content contained in this article is for informational purposes only. The content is not intended to be a substitute for professional advice. Reliance on any information provided in this article is solely at your own risk.
To comment on this article, contact rdavidson@uspharmacist.com.






