Coronavirus disease 2019 (COVID-19) is a respiratory virus that is identified under the severe acute respiratory syndrome (SARS) class. Although most tests for COVID-19 rely on blood samples, noninvasive saliva testing has been regarded as a potential candidate for rapid and accurate SARS coronavirus 2 (SARS-CoV-2) testing.1 This article provides an overview of the COVID-19 virus and the rationale for saliva testing and reviews the benefits and limitations of the saliva-based test SalivaDirect.
Although blood is a primary component for diagnosis, saliva contains various properties that give it the potential to be used as a diagnostic tool. The gland itself is surrounded by capillaries that allow for the exchange of molecules through the blood vessels.2 Proteins, enzymes, antibodies, and cytokines are biomarkers found in blood circulation that flow through the capillaries and can eventually be secreted into the saliva.3 COVID-19 is detected by an antigen test or antibody test which utilizes nasopharngyeal, nasal swab, or blood specimens. Since blood can circulate throughout the salivary gland, it can provide those biomarkers necessary to diagnose COVID-19.4
Saliva testing has been a fundamental diagnostic tool for a plethora of conditions such as human immunodeficiency virus (HIV), autoimmune disorders, and other infectious diseases. Benefits that saliva testing can have over other diagnostic tests include being noninvasive, having more practical storage requirements, and easier sample collection in certain patient populations (e.g., pediatric). Overall, COVID-19 saliva testing can be extremely beneficial to help reduce the spread. It could also be impactful by reducing costs and providing easier access for patients.4
Epidemiology
The novel SARS-CoV-2 first appeared in Wuhan, China, in association with a live-animal market. It is caused by a novel virus that previously has not been seen in humans. The CDC estimates that the United States has more than 1.7 million newly diagnosed COVID-19 cases every week.5 The risk of severe illness from COVID-19 increases based on age. Eight out of ten COVID-19–related deaths reported in the U.S. occurred among adults aged 65 years and older.6 People of any age with the following conditions are at increased risk of severe illness from COVID-19: cancer, chronic kidney disease, chronic obstructive pulmonary disease, immunocompromised state, obesity, serious heart conditions, sickle cell disease, and type 2 diabetes mellitus. There have been over 25 million total confirmed cases and more than 400,000 total deaths in the U.S as of January 27, 2021.5
Incidence is higher in people of color, including Hispanics, African Americans, and Native Americans. Health and social inequalities are factors that contribute to the increased risk of COVID-19 in these ethnic groups. Examples include essential-worker employment status, residence in multigenerational and multifamily households, underlying medical conditions, and limited access to healthcare. Additional socioeconomic factors, such as relying on public transportation, may also contribute to higher levels of exposure to pollution, environmental hazards, and an overall increase in exposure to the COVID-19 virus.7
Etiology
COVID-19 is caused by SARs-CoV-2 infection. It is transmitted primarily through respiratory secretions, such as when an individual sneezes, coughs, or talks. Another mode of transmission occurs when an individual touches a surface with the virus on it, then touches his/her mouth, nose, or eyes.8
There are five stages the virus goes through to replicate. Stage I is the attachment stage where the virus attaches to the host cell. Stage II is the penetration stage. During this stage, the virus enters the host through endocytosis or membrane fusion. Stage III is the biosynthesis stage where viral mRNA makes viral proteins. One of these proteins, known as the spike protein, consists of subunit 1 and 2. Subunit 1 is used to help the virus bind to the host cell, and subunit 2 fuses the viral and cellular membranes. The spike protein attaches to angiotensin-converting enzyme 2 (ACE-2) receptors, a prominent receptor on the lungs. This binding explains why COVID-19 has a significant impact on an individual’s lung function. After binding, the spike protein goes through protease cleavage and is activated at a site adjacent to subunit 2. The virus then proceeds to stage IV, which is the maturation stage at which new viruses are made. Stage V occurs when the new virus is released into the host to continuously repeat this replication cycle.9
Diagnosis
Symptoms associated with a COVID-19 diagnosis include a new onset of fever or chills, accompanied by cough and/or difficulty breathing. Other symptoms may include fatigue, muscle or body aches, headache, new loss of taste or smell, sore throat, congestion or runny nose, nausea or vomiting, and diarrhea. It is important to note that some patients may appear asymptomatic while being carriers of the disease. The likelihood of a COVID-19 diagnosis is increased if the patient under investigation has traveled in the past 14 days to an area with a high prevalence of cases or if the patient has been in contact with other individuals who have a suspected or confirmed diagnosis of COVID-19.10 Selection of a diagnostic test can be based on the cost, test volume, staffing needs, and turnaround time of results. Some of the diagnostic tests include the nasopharyngeal swab, oropharyngeal swab, and saliva sampling.11 The nasopharyngeal swab collection sampling is more diagnostically sensitive compared with the oropharyngeal swab. In one study, the SARS-CoV-2 detection rate was 36.7% higher with the nasopharyngeal swab.12
The CDC prioritizes diagnostic testing for individuals who have symptoms and those who are at high risk for exposure, such as healthcare workers, first responders, hospitalized patients, and residents in long-term-care facilities who present with signs and symptoms.13
According to the Infectious Diseases Society of America, there are multiple methods to confirm a diagnosis of COVID-19. These include “nucleic acid sequencing matching SARS-CoV-2 reference sequences, two positive Nucleic Acid Amplification Test, or NAAT, results, clinical signs and symptoms in addition to documented SARS-CoV-2 seroconversion, and clinical signs and symptoms with a positive index test from two different anatomic sites.”14
Management and Safety Precautions
Safety precautions for the prevention of COVID-19 include maintaining a physical distance of at least 6 feet from others as well as wearing a mask in public.15 Additionally, it is recommended for all individuals to practice hand hygiene by washing hands for at least 20 seconds with soap and warm water. Hand sanitizer, with a minimum of 60% alcohol base, may serve as an alternative when water and soap are unavailable. For vulnerable individuals, such as immunocompromised patients and those with cardiovascular or respiratory disorders, it is recommended to continue their regular treatment regimen.16
Nonpharmacologic treatment for COVID-19 is aimed at treating various complications of the virus. These treatments consist of adequate hydration for coughing, breathing techniques for milder symptoms or mechanical ventilation for severe symptoms, and relaxation techniques for anxiety.17 Multiple investigational drugs and drugs approved for other indications have been studied for the treatment of COVID-19. Remdesivir, an adenosine nucleotide prodrug with antiviral activity, was originally issued an Emergency Use Authorization (EUA) by the FDA for people with severe COVID-19. As of October 22, 2020, this drug was granted full FDA approval under the trade name Veklury, for hospitalized patients who weigh more than 40 kg and are aged 12 years or older.18 An EUA was also issued by the FDA for the use of convalescent plasma for hospitalized patients.19 It is not recommended to use hydroxychloroquine or chloroquine in hospitalized patients with COVID-19. The EUA for hydroxychloroquine and chloroquine was retracted in June of 2020.20 The U.S. Department of Health and Human Services and the Department of Defense developed an agreement with Pfizer Inc. for large-scale production and nationwide delivery of 100 million doses of a COVID-19 vaccine, with the option of an additional 500 million doses. After receiving EUA or licensure from the FDA, this agreement was to be executed and available to American citizens.21 As of press time, the EUA had been received and vaccine distribution is underway.
SalivaDirect
On August 15, 2020, the FDA issued an EUA to Yale School of Public Health for its SalivaDirect COVID-19 diagnostic test.22 This real-time reverse transcription polymerase chain reaction (RT-qPCR) test is a qualitative test designed to detect nucleic acid from SARS-CoV-2 in saliva. Similar to other COVID-19 tests that look for the virus’ nucleic acid, SalivaDirect still requires testing performed in a Clinical Laboratory Improvement Amendments (CLIA)-certified lab. SalivaDirect technology does not require RNA extraction. Although the difficult RNA-extraction step has been removed, the test is still considered complex since it requires polymerase chain reaction (PCR). This is not a rapid test that can be evaluated in proximity to the patient. Therefore, patients still must wait for their results to come back from laboratory testing.22
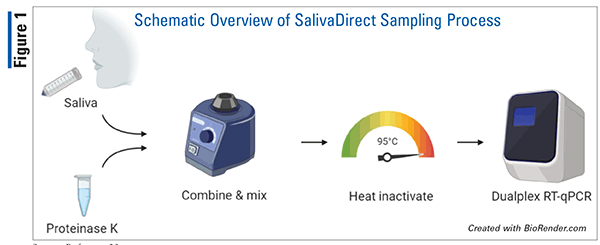
Since SalivaDirect is a protocol, and not a kit, there are no products to distribute. A protocol can be provided to designated laboratories for completing inexpensive saliva-based RT-qPCR testing. The SalivaDirect sampling process is shown in FIGURE 1.23 It consists of three steps: 1) collecting saliva without preservative buffers in a sterile container, 2) proteinase K treatment and heat inactivation, and 3) RT-qPCR virus detection. Compared with other COVID-19 diagnostic tests, saliva-based testing offers several advantages. First, collecting a saliva sample is more tolerable than collecting a sample from a nasopharyngeal swab. Additionally, the removal of the RNA- extraction step means this method is less likely to be affected by supply shortages. This method has been validated with multiple reagents and instruments from various vendors. If supply-chain issues arise, this flexibility will allow for continued testing.24
Saliva as a Diagnostic Tool
Salivary droplets are considered the main source of human-to-human transmission of the virus. Still, before saliva can be used as a diagnostic tool, there must be confirmation of the presence of the virus in saliva. A study was conducted on 25 patients who were admitted to the hospital after diagnosis of severe COVID-19 by real-time reverse (rRT)-PCR. All 25 patients had saliva collected and confirmed positive by rRT-PCR. Furthermore, two of the patients had positive results in the saliva samples while the respiratory swabs showed negative results. Although it must be clarified if the virus is in the saliva or from migration into the mouth from the nasopharynx, this study highlights saliva as a potential tool for diagnosis of COVID-19.25
Another study prospectively compared the detection of SARS-CoV-2 between saliva samples and nasopharyngeal samples in 76 patients. Both sample types were simultaneously collected from patients who were either suspected to have COVID-19 or had a positive diagnosis of COVID-19. The results revealed that the concordance rate of the virus detection between the two samples was as high as 97.4%. Although limited, these results suggest that saliva may be a reliable alternative to nasopharyngeal swabs.26
Limitations
Although SalivaDirect has the potential to serve as an easily accessible and simple-to-use test, limitations do exist. Developers stated that the expected cost would only amount to about $5 per test, but other costs must be considered, such as chemicals, highly skilled labor, and laboratory spending. These additional factors could make the overall cost of saliva testing similar to costs seen in currently used SARS-CoV-2 RNA tests. Additionally, testing would require skilled professional labor because it is a manual PCR test and would require experience with both microbiology and molecular biology to be done correctly. The saliva itself poses some difficulties since it is sticky and hard to collect, has risks for aerosolization or contamination with blood and sputum, and is 10 times to 50 times less sensitive for sampling than a nasopharyngeal swab.27 Therefore, personnel who are performing testing should consider the limitations regarding the reliability of using saliva—for example, if a patient has a low viral loads in their saliva, false-negatives can occur.
Conclusion
COVID-19 is caused by SARS-CoV-2 infection. It is primarily spread through respiratory secretions. Individuals typically present with fever, cough, difficulty breathing, and loss of taste and/or smell within 14 days of exposure. Early detection and diagnosis of COVID-19 can help to limit the spread. The SalivaDirect diagnostic test is a protocol used to determine if an individual has COVID-19 by detecting the RNA of SARS-CoV-2 in saliva. This testing method is simple and does not require preservatives or specialized equipment. For more information regarding the SalivaDirect diagnostic test, visit www.fda.gov/media/141192/download.
The content contained in this article is for informational purposes only. The content is not intended to be a substitute for professional advice. Reliance on any information provided in this article is solely at your own risk.
REFERENCES
1. Czumbel LM, Kiss S, Farkas N, et al. Saliva as a candidate for COVID-19 diagnostic testing: a meta-analysis. Front Med. 2020;7:465.
2. Zhang CZ, Cheng XQ, Li JY, et al. Saliva in the diagnosis of diseases. Int J Oral Sci. 2016;8(3):133-137.
3. Tiwari M. Science behind human saliva. J Nat Sci Biol Med. 2011;2(1):53-58.
4. Sri Santosh T, Parmar R, Anand H, et al. A review of salivary diagnostics and its potential implication in detection of Covid-19. Cureus. 2020;12(4):e7708. April 17, 2020.
5. CDC. COVID data tracker. Updated January 6, 2021. https://covid.cdc.gov/covid-data-tracker/#cases_casesinlast7days. Accessed January 12, 2021.
6. CDC. Older adults and COVID-19. Updated December 13, 2020. www.cdc.gov/coronavirus/2019-ncov/need-extra-precautions/older-adults.html. Accessed January 12, 2021.
7. Moore JT, Ricaldi JN, Rose CE, et al. Disparities in incidence of COVID-19 among underrepresented racial/ethnic groups in counties identified as hotspots during June 5–18, 2020—22 States, February–June 2020. MMWR Morb Mortal Wkly Rep. 2020;69(33):1122-1126.
8. World Health Organization. Transmission of SARS-CoV-2: implications for infection prevention precautions. Published July 9, 2020. www.who.int/news-room/commentaries/detail/transmission-of-sars-cov-2-implications-for-infection-prevention-precautions. Accessed January 27, 2021.
9. Yuki K, Fujiogi M, Koutsogiannaki S. COVID-19 pathophysiology: a review. Clin Immunol. 2020;215:108427.
10. CDC. Symptoms of coronavirus. Published December 22, 2020. www.cdc.gov/coronavirus/2019-ncov/symptoms-testing/symptoms.html. Accessed January 12, 2021.
11. Fang FC, Naccache SN, Greninger AL. The laboratory diagnosis ofcoronavirus disease 2019—frequently-asked questions. Clin Infect Dis. 2020;71(11):2996-3001.
12. Wang H, Liu Q, Hu J, et al. Nasopharyngeal swabs are more sensitive than oropharyngeal swabs for COVID-19 diagnosis and monitoring the SARS-CoV-2 load. Front Med. 2020;7:334.
13. National Institutes of Health. COVID-19 treatment guidelines Panel. Updated December 17, 2020. Coronavirus disease 2019 (COVID-19) treatment guidelines. www.covid19treatmentguidelines.nih.gov/. Accessed January 12, 2021.
14. Infectious Diseases Society of America. Infectious Diseases Society of America guidelines on the diagnosis of COVID-19: molecular diagnostic testing. December 23, 2020. www.idsociety.org/practice-guideline/covid-19-guideline-diagnostics/. Accessed January 12, 2021.
15. CDC. How to protect yourself & others. Updated December 31, 2020. www.cdc.gov/coronavirus/2019-ncov/prevent-getting-sick/prevention.html?CDC_AA_refVal=https%3A%2F%2Fwww.cdc.gov%2Fcoronavirus%2F2019-ncov%2Fneed-extra-precautions%2Fwhat-you-can-do.html. Accessed January 12, 2021.
16. CDC. If you are immunocompromised, protect yourself from COVID-19. Updated December 16, 2020. www.cdc.gov/coronavirus/2019-ncov/need-extra-precautions/immunocompromised.html. Accessed January 12, 2021.
17. Bajwah S, Wilcock A, Towers R, et al. Managing the supportive care needs of those affected by COVID-19. Eur Respir J. 2020;55(4):2000815.
18. FDA. FDA approves first treatment for COVID-19. Published October 22, 2020. www.fda.gov/news-events/press-announcements/fda-approves-first-treatment-covid-19. Accessed October 26, 2020.
19. National Institutes of Health. COVID-19 treatment guidelines. 2020. What’s new in the guidelines. Updated December 17, 2020. www.covid19treatmentguidelines.nih.gov/whats-new/. Accessed January 12, 2021.
20. FDA. Frequently asked questions on the emergency use authorization for chloroquine phosphate and hydroxychloroquine sulfate. Updated December 16, 2020. www.fda.gov/media/136784/download. Accessed January 12, 2021.
21. U. S. Department of Health and Human Services. U.S. Government engages Pfizer to produce millions of doses of COVID-19 Vaccine. Published July 22, 2020. www.hhs.gov/about/news/2020/07/22/us-government-engages-pfizer-produce-millions-doses-covid-19-vaccine.html. Accessed September 20, 2020.
22. FDA. Coronavirus (COVID-19) update: FDA issues emergency use authorization to Yale School of Public Health for SalivaDirect, which uses a new method of saliva sample processing. Published August 15, 2020. www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-issues-emergency-use-authorization-yale-school-public-health. Accessed September 14, 2020.
23. Yale School of Public Health. SalivaDirect™. https://publichealth.yale.edu/SalivaDirect. Accessed September 21, 2020.
24. Vogels CB, Brackney DE, Wang J, et al. SalivaDirect: Simple and sensitive molecular diagnostic test for SARS-CoV-2 surveillance. Published August 4, 2020. medRxiv.com. www.medrxiv.org/content/10.1101/2020.08.03.20167791v1.full.pdf. Accessed January 8, 2020.
25. Azzi L, Carcano G, Gianfagna F. Saliva is a reliable tool to detect SARS-CoV-2. J Infect. 2020;81(1):e45-e50.
26. Iwasaki S, Fujisawa S, Nakakubo S, et al. Comparison of SARS-CoV-2 detection in nasopharyngeal swab and saliva. J Infect. 2020; 81(2):e145-e147.
27. ASM.org. What is the COVID-19 SalivaDirect test? Published August 25, 2020. https://asm.org/Articles/2020/August/What-is-the-COVID-19-SalivaDirect-Test. Accessed September 19, 2020.
To comment on this article, contact rdavidson@uspharmacist.com.