US Pharm. 2014;39(1):HS6-HS10.
ABSTRACT: Spinal cord injuries (SCIs) are the result of a trauma to the spinal cord that causes a change in normal motor, sensory, or autonomic function. These injuries require a comprehensive approach to pharmacologic treatment in the acute setting. In 2013, an estimated 273,000 U.S. patients had some form of SCI. Respiratory management consists of prevention and treatment of pneumonia and the possibility of respiratory support through intubation. Hemodynamic instability and dysreflexia must be identified early and treated in response to the causative factor. Anticoagulation should be started as necessary for venous thromboembolism and continued for the appropriate duration. Therapy options for pain and spasticity, which are difficult to successfully treat, include opioids, antidepressants, and baclofen.
Spinal cord injuries (SCIs) are the result of a trauma to any area of the spinal cord that alters normal motor, sensory, or autonomic function. The average age at injury is 42.6 years. The estimated annual incidence is 40 cases per million, or 12,000 new cases annually. The estimated prevalence is 273,000 persons (range 238,000-332,000), with 80.7% males and 19.3% females.1 In 2010, causes of SCI were motor vehicle crashes (37%), falls (29%), violence (14%), sports (9%), and other/unknown (11%).1
In the past, renal failure was the leading cause of death in patients with SCI; however, thanks to advances in urologic management, this is no longer the case.1,2 Currently, pneumonia and septicemia appear to have the greatest impact on reducing life expectancy.1,2 Leading secondary complications after SCI include pressure sores, chills and fever secondary to urinary sepsis, atelectasis, pneumonia, and thromboembolism, all of which often necessitate rehospitalization.2
Pathophysiology of SCI
The severity of neurologic deficit after injury depends upon the area of injury and the extent of the lesion(s). Recovery of neurologic function is dramatically affected, with fewer than 1% of SCI patients experiencing complete neurologic recovery by hospital discharge. Recovery in those with incomplete injuries is related to the severity of the initial neurologic deficit.2
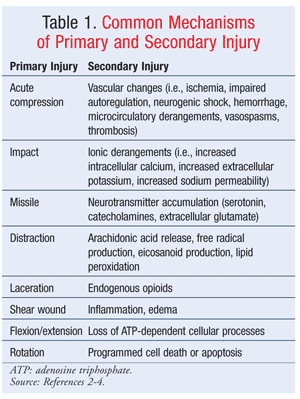
In acute SCI, many biomolecular changes occur in a two-step process that consists of primary and secondary mechanisms (TABLE 1).2-4 The primary injury mechanism relates to the initial mechanical injury.2-4 This injury most commonly is due to the combination of local deformation caused by the initial impact and subsequent persisting compression and energy transformation.2,3 The secondary injury mechanism is the result of the primary mechanism initiating a cascade of biochemical and cellular processes that cause ongoing cellular damage and cell death.2,3 Pathways implicated in mediating this secondary mechanism include apoptosis, intracellular protein synthesis inhibition, and glutaminergic mechanisms.2,3
Pharmacologic Therapies
Respiratory Support: SCI most often results in alterations in cardiopulmonary function, requiring the patient to be monitored in the ICU. Leading causes of mortality in patients with SCI include pneumonia, atelectasis, and other respiratory complications.5 The risk of these complications increases with increased injury severity.5 Paralysis of respiratory muscles causes poor mobilization against bacteria and accumulated secretions, which may lead to respiratory infections.5 If the patient requires intubation, the choice of induction and neuromuscular agents must be carefully considered. Propofol and thiopental may not be ideal in hemodynamically unstable patients because of exacerbation of hypotension caused by hemorrhage, neurogenic shock, and sepsis.6 The use of ketamine and etomidate is controversial owing to their possible unwanted effects: Ketamine may cause hypertension and elevate intracranial pressure, specifically in patients with head injury, and etomidate is a concern in critically ill patients because of its ability to inhibit adrenal steroid synthesis and cause hypotension, resulting in the need for vasopressors.6 In terms of neuromuscular blocking agents, succinylcholine remains the drug of choice, but only when used within the first 48 hours of injury, because of the risk of precipitating hyperkalemia.6,7
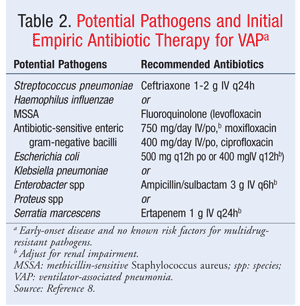
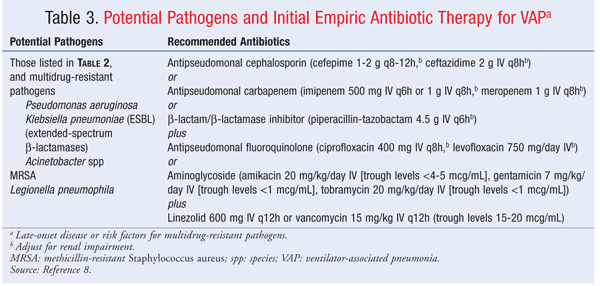
Pneumonia: The type of pneumonia most commonly seen in SCI patients is ventilator-associated pneumonia (VAP). VAP is defined as the onset of pneumonia occurring 48 to 72 hours following endotracheal intubation and mechanical ventilation.7,8 Early-onset VAP occurs in the first 4 days of intubation; late-onset VAP occurs after 4 days of intubation.7,8 Potential pathogens and initial empiric antibiotic therapy for patients with early-onset disease and no known risk factors for multidrug resistance are given in TABLE 2. TABLE 3 lists potential pathogens and initial empiric antibiotic therapy for patients with late-onset disease or risk factors for multidrug resistance. Empiric antibiotics for treatment of VAP should be selected to cover the suspected pathogens until final cultures result, and then de-escalated as appropriate.8 Short-acting and long-acting beta-agonist bronchodilators, hydrating agents, and mucolytics also may be used to reduce respiratory complications and improve respiratory function.5 The use of ipratropium in SCI patients is controversial, since it contains an atropine analogue and can block the release of surfactant essential for prevention and treatment of atelectasis.5 Agents such as cromolyn sodium and methylxanthines may be considered, but their use is questionable owing to the lack of studies in SCI patients.5
Hemodynamic Instability/Neurogenic Shock and Autonomic Dysreflexia: Hemodynamic instability or neurogenic shock often occurs with acute SCI, resulting in hypotension and cardiac arrhythmias such as bradycardia, supraventricular tachycardia, and ventricular tachycardia.6,7 Arrhythmias are most common in the first 14 days after injury and in severe injuries.6,7 Hypotension results from a loss of vasoconstrictor tone in the peripheral arterioles, with consequent pooling of blood in the peripheral vasculature.6,7 Volume resuscitation is first-line treatment when all other causes of hypotension have been ruled out.4,6,7,9 If volume resuscitation is not successful, a vasopressor with both alpha- and beta-adrenergic activity, such as dopamine or norepinephrine, should be used to counter the loss of sympathetic tone and provide chronotropic support.4,6,7,9 Patients experiencing bradycardia should be treated with atropine as appropriate.4,9
Patients may also experience autonomic dysreflexia (AD; also called autonomic hyperreflexia), which is characterized by sudden, potentially dangerous elevations in blood pressure (BP) resulting from various noxious stimuli triggering sympathetic hyperactivity after spinal shock.4 The most common causes of AD are bladder and bowel distention; however, any painful or irritating stimulus below the area of injury can precipitate AD.4,10 Signs and symptoms include BP 20 to 40 mmHg above baseline associated with bradycardia, headache, profuse sweating above the area of injury, cardiac arrhythmias, goose bumps, skin flushing, blurred vision and/or altered visual field, nasal congestion, and anxiety.4,10 Although these findings are common, some patients with elevated BP experience minimal or no symptoms, a condition known as silent autonomic dysreflexia.10
Pharmacologic treatment of high BP should be considered when the systolic BP is 150 mmHg or higher.10 Nitroglycerin ointment 2% may be used, with 1 inch applied to the skin above the level of SCI.10 In a monitored setting, sodium nitroprusside and nitroglycerin IV drip may be used to achieve rapid titration of BP.10 Other agents, such as hydralazine, diazoxide, and phenoxybenzamine, have been used to treat severe symptoms caused by AD.10 Prazosin and captopril also have been used for BP control.10
Neuroprotection: Much controversy exists concerning the use of neuroprotective agents in SCI. To date, there is no clinical evidence to definitively recommend their use to preserve or improve spinal cord function after injury.3,6,11-13 Methylprednisolone, GM1 ganglioside, gacyclidine, tirilazad, and naloxone have been studied in large-scale, multicenter clinical trials (with methylprednisolone and GM1 ganglioside studied the most).3,6,11 However, when combined, these studies show conflicting results, and complete evaluation of the literature is beyond the scope of this article.6,11-13 The use of any of these agents should be determined after careful consideration of the risk-versus-benefit profile, the risk of severe side effects, and the lack of alternative therapies.3,6,11-13
Thromboembolism: Frequently seen in SCI, thromboembolism may be prevented via chemical or mechanical methods. The recommended treatment of venous thromboembolism (VTE) uses a combination of methods that includes low-dose heparin or low-molecular-weight heparins. Examples of medications and dosages used for VTE prophylaxis appear in TABLE 4. Early recognition of prophylactic need and administration of prophylaxis within 72 hours of admission should be a goal. The recommended duration of chemical prophylaxis for deep venous thrombosis (DVT) is at least 3 months. If a patient regains lower-limb function or mobility, a shorter duration may be used. The rationale for 3 months stems from data showing that most DVTs occur within this time frame.14
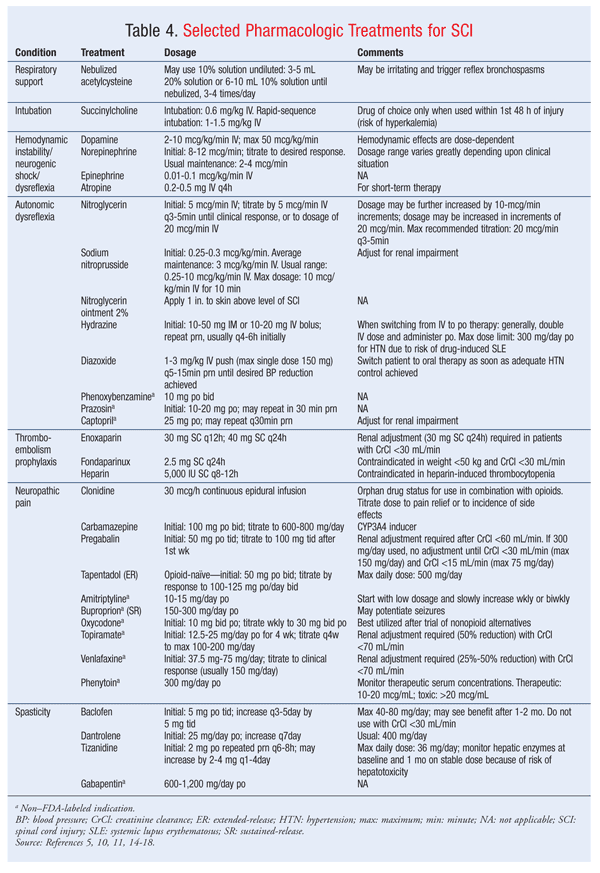
Pain: Pain associated with SCI is varied in symptoms and management. The onset of pain may present shortly after the inciting injury and remain indefinitely. Neuropathic pain may present above (uncommonly) or below the SCI site. SCI pain is usually treated with medications used to treat neuropathic pain. Drug classes utilized for neuropathic SCI-related pain, with varying degrees of effectiveness, include antidepressants, anticonvulsants, nonsteroidal anti-inflammatory drugs, sodium channel blockers, clonidine, and opioids. TABLE 4 gives examples of medications and dosages used for neuropathic pain. Other routes of pain management include behavioral and physical therapy regimens shown to possibly improve pain control in combination with pharmacologic agents.15,16 The pharmacologic management of SCI pain is often augmented with surgery (e.g., dorsal root entry zone ablation) and is usually indicated only for neuropathic pain with complete SCI.17 Therapy should be individualized to maximize pain control.
Spasticity: Spasticity after SCI is a common occurrence that requires maximal integration of surgical and pharmacologic options. Baclofen and diazepam, both of which have been shown to provide relief from spasticity, focus primarily on inhibition of central nervous stimulation by facilitating gamma-aminobutyric acid receptors. Both of these agents are limited by their side-effect profile—most notably, sedation. Dantrolene has a unique mechanism that acts on the skeletal muscle and reduces contractions by modulating calcium release. Muscle weakness is the main limiting side effect, and patients should also be monitored for hepatotoxicity. Tizanidine and gabapentin also may be beneficial.6 Examples and dosing of medications used for spasticity are found in TABLE 4.
Conclusion
Although no treatment currently exists to reverse the neurologic deficit resulting from SCI, it is necessary to manage secondary complications. The main areas for medical management include respiratory problems, neurogenic shock, autonomic dysreflexia, thromboembolism, pain, and spasticity. Definitive recommendations for use of neuroprotective agents are lacking, and further research is needed in this area. SCI treatment in the acute and chronic settings presents unique and challenging pharmacologic issues that the pharmacist can address as part of the healthcare team. Understanding the sequelae of SCI allows the pharmacist to accurately manage the patient’s medications and improve his or her quality of life after SCI.
REFERENCES
1. National Spinal Cord Injury Statistical Center. Facts and Figures at a Glance. Birmingham, AL: University of Alabama at Birmingham; March 2013.
2. Sekhon LH, Fehlings MG. Epidemiology, demographics, and pathophysiology of acute spinal cord injury. Spine (Phila PA 1976). 2001;26(suppl 24):S2-S12.
3. Kwon BK, Tetzlaff W, Grauer JN, et al. Pathophysiology and pharmacologic treatment of acute spinal cord injury. Spine J. 2004;4:451-464.
4. McMahon D, Tutt M, Cook AM. Pharmacological management of hemodynamic complications following spinal cord injury. Orthopedics. 2009;32:331.
5. Consortium for Spinal Cord Medicine. Respiratory management
following spinal cord injury: a clinical practice guideline for
health-care professionals. J Spinal Cord Med. 2005;28:259-293.
6. Consortium for Spinal Cord Medicine. Early acute management in
adults with spinal cord injury: a clinical practice guideline for
health-care professionals. J Spinal Cord Med. 2008;31:408-479.
7. Ball PA. Critical care of spinal cord injury. Spine (Phila PA 1976). 2001;26(suppl 24):S27-S30.
8. American Thoracic Society; Infectious Diseases Society of America.
Guidelines for the management of adults with hospital-acquired,
ventilator-associated, and healthcare-associated pneumonia. Am J Respir Crit Care Med. 2005;171:388-416.
9. Nockels RP. Nonoperative management of acute spinal cord injury. Spine (Phila Pa 1976). 2001;26(suppl 24):S31-S37.
10. Consortium for Spinal Cord Medicine. Acute management of
autonomic dysreflexia: individuals with spinal cord injury presenting to
health-care facilities. J Spinal Cord Med. 2002;25(suppl 1):S67-S88.
11. Hurlbert RJ, Hadley MN, Walters BC, et al. Pharmacological therapy for acute spinal cord injury. Neurosurgery. 2013;72(suppl 2):93-105.
12. Sayer FT, Kronvall E, Nilsson OG. Methylprednisolone treatment in
acute spinal cord injury: the myth challenged through a structured
analysis of published literature. Spine J. 2006;6:335-343.
13. Fehlings MG; Spine Focus Panel. Summary statement: the use of methylprednisolone in acute spinal cord injury. Spine (Phila Pa 1976). 2001;26(suppl 24):S55.
14. Dhall SS, Hadley MN, Aarabi B, et al. Deep venous thrombosis and
thromboembolism in patients with cervical spinal cord injuries. Neurosurgery. 2013;72(suppl 2):244-254.
15. Burchiel KJ, Hsu FP. Pain and spasticity after spinal cord injury: mechanisms and treatment. Spine (Phila Pa 1976). 2001;26(suppl 24):S146-S160.
16. Mehta S, Orenczuk K, McIntyre A, et al; SCIRE Research Team.
Neuropathic pain post spinal cord injury part 1: systematic review of
physical and behavioral treatment. Top Spinal Cord Inj Rehabil. 2013;19:61-77.
17. Mehta S, Orenczuk K, McIntyre A, et al; SCIRE Research Team.
Neuropathic pain post spinal cord injury part 2: systematic review of
dorsal root entry zone procedure. Top Spinal Cord Inj Rehabil. 2013;19:78-86.
18. Clinical Pharmacology [subscription database]. www.clinicalpharmacology.com. Accessed November 29, 2013.
To comment on this article, contact rdavidson@uspharmacist.com.





