US Pharm. 2019;44(11):HS8-HS12.
ABSTRACT: Alcohol-withdrawal syndrome (AWS) is a challenge to patient care that can present in the inpatient setting. Early identification and treatment initiation in patients with a history of alcohol-use disorder are necessary in order to minimize the development of AWS. Evidence supports the use of benzodiazepines via various dosing strategies and the addition of supportive and nutritional care to mitigate withdrawal symptoms. Other possible treatment options for AWS include barbiturates, anticonvulsants, and adrenergic medications, which vary in terms of their benefit. The pharmacist can assist the multidisciplinary team in identifying patients at risk for AWS as well as recommend safe and effective treatment regimens on an individual basis.
The World Health Organization estimates that 283 million people globally have alcohol-use disorder (AUD), which comprises alcohol dependence and alcohol abuse.1,2 This population presents with significant complications that are associated with physical and psychiatric comorbidities. AUD contributes to morbidity and mortality worldwide.3,4
AUD is primarily considered a chronic condition that is most significant in the outpatient setting, but clinicians in inpatient settings also face AUD-related challenges. Although patients may initially present for conditions unrelated to AUD, sudden reduction or cessation of alcohol consumption upon hospitalization can put patients at risk for alcohol-withdrawal syndrome (AWS). AWS symptoms, including anxiety, agitation, irritability, confusion, tremor, and hemodynamic changes, can complicate a patient’s clinical course.5,6
The severity of alcohol-withdrawal symptoms is extremely variable between patients, ranging from mild anxiety to major seizures. The most severe cases may develop into delirium tremens (DT), a severe psychotic condition involving acute confusion, hallucinations, and tremors. Early identification, risk assessment, and treatment of patients with known AUD are necessary for minimizing the negative outcomes associated with AWS and preventing development of symptoms that would further complicate a patient’s hospital visit.5-7
Pathophysiology
Alcohol, a central nervous system (CNS) depressant, exerts its effect primarily by altering the neurochemical balance of the brain. This occurs through an increase in the inhibitory effects of the gamma-aminobutyric acid (GABA) pathway and suppression of the excitatory neurotransmitter glutamate, specifically through binding to the N-methyl-d-aspartate (NMDA) receptor. Chronic alcohol use can result in adaptive changes to the neurochemical balance of the brain. To recover homeostasis, a downregulation of GABA-associated receptors and an upregulation of glutamate-associated NMDA receptors occur, leading to a decrease in the CNS effects of alcohol use, which results in tolerance.5,8-11
These neurochemical changes may go undetected in patients with prolonged alcohol use; however, upon reduction or cessation of alcohol consumption, serious CNS effects can occur. Without the direct effect of alcohol on the neurotransmitter systems, a dramatic decrease in the inhibitory GABA pathway and increase in the excitatory glutamate-mediated pathway take place. This acute imbalance can result in the CNS effects commonly associated with AWS, including delirium, hallucinations, and decreased seizure threshold.5,8-10
Diagnosis and Screening
Initial evaluation of a patient with AWS relies heavily on clinical presentation. Diagnosis is based mainly on symptoms and may be guided by the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5). DSM-5 criteria for AWS include presentation with any two identified symptoms, including autonomic symptoms (diaphoresis, tachycardia), increased hand tremors, nausea and/or vomiting, psychomotor agitation, anxiety, generalized tonic-clonic seizures, and hallucinations.2,12
Obtaining a medical and social history is also necessary for diagnosis and to rule out other concurrent conditions with presentations similar to AWS. Historical data including quantity of alcohol ingested, duration of alcohol use, time since last drink, history of alcohol withdrawal, abuse of other agents, and concurrent medical or psychological problems can provide important context for individual patient cases. Laboratory data such as metabolic panels, blood-alcohol levels, liver-function tests, and toxicology screens may be useful, especially when the provider is unable to obtain an adequate patient history.6,13-15
Although several assessment tools are available to aid in diagnosis, the Clinical Institute Withdrawal Assessment for Alcohol, Revised (CIWA-Ar), is the most commonly used tool.16-18 CIWA-Ar, a 10-item survey that measures the severity of alcohol withdrawal, requires minimal patient participation. Symptoms assessed include nausea and vomiting; tremor; paroxysmal sweats; anxiety; agitation; tactile, auditory, and visual disturbances; headache or fullness in head; and orientation and clouding of sensorium. Most items are rated on a scale of 0 to 7, with 0 being normal and 7 being extremely severe. The cumulative score, which can be a maximum of 67, corresponds to the severity of the patient’s withdrawal. A cumulative score of 1 to 7 indicates mild withdrawal, 8 to 15 denotes moderate withdrawal, and 16 or higher means severe withdrawal.16-19 Institutions frequently use CIWA-Ar in standardized protocols in order to help treat AWS.
Treatment
Overall therapy goals for the AWS patient include minimizing and treating milder symptoms of withdrawal while preventing progression to alcoholic hallucinosis (alcohol-related psychosis), seizures, or DT. Supportive care and benzodiazepines (BZDs) are the mainstays of therapy to achieve these aims, with most evidence supporting the use of BZDs to treat withdrawal.5,6,11,20 Additional treatment options include barbiturates, anticonvulsants, and adrenergics.5,11,20
Supportive Care
Supportive care includes addressing nutritional deficits, treating dehydration, and preventing delirious patients from harming themselves or others. In patients with abnormal vital signs or underlying comorbidities, these factors should be monitored and stabilized as much as possible on presentation. Patients with a long history of AUD often present with malnutrition, which is attributable to poor dietary intake and malabsorption in the gastrointestinal (GI) tract. Chronic alcoholism is associated with a high risk of thiamine deficiency. Thiamine and glucose should be administered to patients experiencing or at high risk for Wernicke encephalopathy, a neurologic condition caused by chronic thiamine deficiency.15,20,21
The standard recommendation is to administer thiamine 100 mg IV prior to the glucose, but the necessity of this order of administration is currently under debate.21 It had been theorized that administering glucose first might precipitate the development of Wernicke encephalopathy, but recent studies have suggested that when the thiamine is administered with or shortly after glucose, the risk is alleviated and necessary treatment of hypoglycemia—if present—may be initiated sooner.15
Additional nutritional deficits and imbalances, such as folic acid, magnesium, and other vitamins and minerals, may exist in patients with chronic AUDs. Literature concerning the overall impact of correcting these deficiencies is conflicting, but enteral or parenteral multivitamins and magnesium may be administered for replacement if appropriate.22 Water and electrolyte disturbances should be corrected as well. Caution is important when rehydrating dehydrated patients because AWS patients may retain excess fluid, leading to fluid overload.13 Physical restraints should generally be avoided, except when necessary to protect the patient or caregivers from harm.22
Pharmacologic Management
Initiation of pharmacologic management of acute alcohol withdrawal is generally based on clinical judgment. In the literature, initiation of pharmacologic therapy is usually recommended only in cases of moderate or severe withdrawal (as designated by a CIWA-Ar score of 8 or higher).6,20 However, clinicians may deem it is also warranted in patients with a low risk of AWS.
BZDs: These agents have the most evidence supporting their efficacy; therefore, they are the most commonly used medication class for treating alcohol withdrawal.5,6,11 BZDs stimulate GABA type A receptors and have been shown to lessen withdrawal severity, including reduced risks of seizures and DT development.20
Evidence suggests that all BZDs are effective for treating AWS.23 Therefore, agents may be selected according to the patient’s specific clinical situation. This decision should be based on various differentiating factors in the drugs’ pharmacokinetic profiles, primarily duration of action and metabolism (TABLE 1). In general, BZDs with active metabolites offer a longer duration of action and may result in fewer rebound CNS effects. These longer-acting agents may be inappropriate for some patients, however, such as those with known hepatic disease or at high risk for respiratory depression.5,20
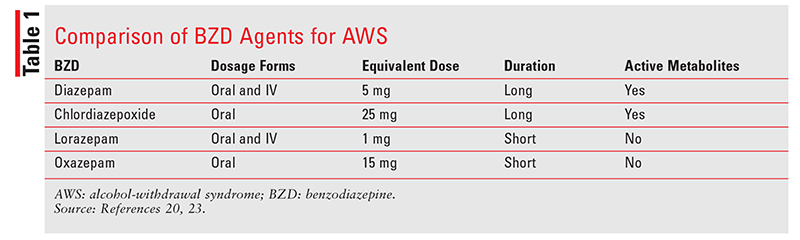
Strategies for BZD therapy include loading-dose, fixed-dose, and symptom-triggered regimens. Loading-dose regimens involve administering a high dose of a long-acting BZD every 2 hours and monitoring the patient’s CIWA-Ar score before every dose until withdrawal symptoms resolve or the patient is undersedated. A reduced dose may then be administered less frequently on an as-needed basis to maintain the desired effect. This regimen may be preferable, as the duration of therapy is shorter (often not more than 1-2 days, although it may be extended to 72 hours if there is a risk of DT) and the overall dose of BZDs required is lowered.15,21 Loading-dose regimens may be especially beneficial in patients experiencing severe withdrawal when the risk of withdrawal symptoms outweighs the risk of oversedation. Patients at higher risk for respiratory depression, such as those who are elderly or have concomitant hepatic or respiratory disorders, may be poor candidates for this approach.5,11,20
Fixed-dose strategies are less aggressive and more patient-specific. An initial total daily dose of BZDs may be determined based on a patient’s average daily alcohol consumption (see BOX 1 for formula).24 Every 10 grams of alcohol are equivalent to one drink and should be countered with 5 mg of diazepam. This dose should then be adjusted for patient comorbidities, risk of accumulation, and amount of time since the patient’s last drink. Long-acting BZDs are recommended owing to the low risk of breakthrough symptoms, although individual patient assessment should dictate the most appropriate agent. The dose is then tapered at scheduled intervals based on continued monitoring, which results in an extended therapy duration compared with loading-dose regimens. This approach is beneficial when barriers to close monitoring exist or CIWA-Ar scores are difficult to determine.5,11,20

Symptom-triggered dosing, a more reactive approach, requires the most monitoring and is based specifically on CIWA-Ar score. This regimen is not appropriate for all patients, but it may reduce the risk of overmedicating. It is not recommended for use in any patient who has previously experienced withdrawal seizures or DT. The patient must also be capable of reporting any withdrawal symptoms being experienced. Although symptom-triggered therapy may have limited applicability, it has demonstrated a reduced duration of therapy and lower cumulative dose compared with fixed-dose regimens.25 Patients without a history of previous withdrawal complicated by seizures or DT who have a moderate withdrawal risk according to CIWA-Ar scores may benefit from this regimen.5,11,20
Barbiturates: Phenobarbital has been investigated as a treatment option for AWS. The potential mechanism for its benefit is a direct effect on the GABA neurochemical pathway that offers a cross-tolerance effect with alcohol. Research on the use of phenobarbital for AWS has historically produced mixed results.20,26,27 However, recent literature has yielded more encouraging data. A retrospective cohort study of ICU patients evaluated treatment with a symptom-triggered BZD regimen versus phenobarbital.26 The phenobarbital cohort demonstrated significantly shorter ICU stays and overall hospitalization than the standard-of-care BZD cohort. The phenobarbital cohort also required fewer intubations and adjunctive agents for further symptom control. It was concluded that phenobarbital may be an effective alternative to traditional BZD therapy.26
Anticonvulsants: Some anticonvulsant agents may also be useful for the management of AWS. Anticonvulsants have been shown to reduce cravings and treat mood disorders, both of which may occur in AWS patients. Although anticonvulsants’ side-effect profiles vary by agent, they are generally less sedating than BZDs, which may be an advantage in some situations.20,28
Carbamazepine (CBZ) is one of the most investigated anticonvulsants for AWS, and research suggests that CBZ may be a suitable alternative to traditional BZD therapy. A study that compared CBZ with lorazepam in an outpatient setting concluded that, in addition to being effective, CBZ was superior for preventing rebound withdrawal symptoms.29 Although CBZ appears to be useful for treatment of AWS, evidence is inconclusive regarding its efficacy in treating withdrawal-associated seizures and DT compared with BZDs.30 This deficit, combined with associated side effects and drug interactions, has generally limited the use of CBZ in this setting.
Other anticonvulsants have also been evaluated, including valproic acid, gabapentin, and vigabatrin. Although findings are more limited than for BZDs and CBZ, all of these agents have demonstrated benefit in AWS and could be considered alternative therapies. Each agent’s adverse-effect profile should be taken into account, especially those of valproic acid. Although valproic acid may be effective, its use is generally limited by side effects that mimic the CNS and GI disturbances that commonly occur in AWS.20,28-33
Adrenergic Drugs: Both adrenergic agonists (e.g., clonidine) and adrenergic antagonists (e.g., propranolol) have been used to treat symptoms of AWS. The benefit is theorized to be linked to reductions in blood pressure and heart rate, which lead to an overall decrease in autonomic response. Evidence generally does not support the use of adrenergic medications for prevention or treatment of withdrawal-associated delirium or seizures. The use of adrenergic medications is limited to adjunctive therapy with BZD treatment, and monotherapy should not be used in patients whose CIWA-Ar category exceeds low risk.20,34
The Pharmacist’s Role
There are many areas for pharmacist intervention with AUD patients in the inpatient setting. Obtaining a thorough medical and social history and medication reconciliation can help clinicians identify patients at risk for withdrawal before symptoms present. Pharmacists can also help the multidisciplinary team select the most appropriate BZD agent and dosing regimen. Based on the status of non-BZD treatments for AWS, pharmacists can provide insight into the appropriateness of adjunct medications for individual patients. Pharmacists can also guide and monitor patients’ prescribed medication therapy throughout the hospital stay and counsel them about their medication at discharge.
REFERENCES
1. World Health Organization. Global status report on alcohol and health 2018. Geneva, Switzerland: World Health Organization; 2018. License: CC BY-NC-SA 3.0 IGO.
2. Grant BF, Goldstein RB, Saha TD, et al. Epidemiology of DSM-5 alcohol use disorder: results from the National Epidemiologic Survey on Alcohol and Related Conditions III. JAMA Psychiatry. 2015;72(8):757-766.
3. Kendler KS, Ohlsson H, Sundquist J, Sundquist K. Alcohol use disorder and mortality across the lifespan: a longitudinal cohort and co-relative analysis. JAMA Psychiatry. 2016;73(6):575-581.
4. Rehm J, Gmel G, Sempos CT, Trevisan M. Alcohol-related morbidity and mortality. Alcohol Res Health. 2003;27(1):39-51.
5. Jesse S, Bråthen G, Ferrara M, et al. Alcohol withdrawal syndrome: mechanisms, manifestations, and management. Acta Neurol Scand. 2017;135(1):4-16.
6. Mirijello A, D’Angelo C, Ferrulli A, et al. Identification and management of alcohol withdrawal syndrome. Drugs. 2015;75(4):353-365.
7. Muzyk AJ, Rogers RE, Dighe G, et al. Impact of an alcohol withdrawal treatment pathway on hospital length of stay: a retrospective observational study comparing pre and post pathway implementation. J Psychiatr Pract. 2017;23(3):233-241.
8. Becker HC, Mulholland PJ. Neurochemical mechanisms of alcohol withdrawal. Handb Clin Neurol. 2014;125:133-156.
9. Wood E, Albarqouni L, Tkachuk S, et al. Will this hospitalized patient develop severe alcohol withdrawal syndrome? The rational clinical examination systematic review. JAMA. 2018;320(8):825-833.
10. McKeon A, Frye MA, Delanty N. The alcohol withdrawal syndrome. J Neurol Neurosurg Psychiatry. 2008;79(8):854-862.
11. Wackernah RC, Minnick MJ, Clapp P. Alcohol use disorder: pathophysiology, effects, and pharmacologic options for treatment. Subst Abuse Rehabil. 2014;5:1-12.
12. O’Malley GF, O’Malley R. Alcohol toxicity and withdrawal. www.merckmanuals.com/professional/special-subjects/recreational-drugs-and-intoxicants/alcohol-toxicity-and-withdrawal. Accessed October 11, 2019.
13. Harris PS, Roy SR, Coughlan C, et al. Chronic ethanol consumption induces mitochondrial protein acetylation and oxidative stress in the kidney. Redox Biol. 2015;6:33-40.
14. Bayard M, McIntyre J, Hill KR, Woodside J Jr. Alcohol withdrawal syndrome. Am Fam Physician. 2004;69(6):1443-1450.
15. Kattimani S, Bharadwaj B. Clinical management of alcohol withdrawal: a systematic review. Ind Psychiatry J. 2013;22(2):100-108.
16. Wetterling T, Weber B, Depfenhart M, et al. Development of a rating scale to predict the severity of alcohol withdrawal syndrome. Alcohol Alcohol. 2006;41(6):611-615.
17. Maldonado JR, Sher Y, Ashouri JF, et al. The “Prediction of Alcohol Withdrawal Severity Scale” (PAWSS): systematic literature review and pilot study of a new scale for the prediction of complicated alcohol withdrawal syndrome. Alcohol. 2014;48(4):375-390.
18. Sullivan JT, Sykora K, Schneiderman J, et al. Assessment of alcohol withdrawal: the revised clinical institute withdrawal assessment for alcohol scale (CIWA-Ar). Br J Addict. 1989;84(11):1353-1357.
19. Eloma AS, Tucciarone JM, Hayes EM, Bronson BD. Evaluation of the appropriate use of a CIWA-Ar alcohol withdrawal protocol in the general hospital setting. Am J Drug Alcohol Abuse. 2018;44(4):418-425.
20. Sachdeva A, Choudhary M, Chandra M. Alcohol withdrawal syndrome: benzodiazepines and beyond. J Clin Diagn Res. 2015;9(9):VE01-VE07.
21. Watson AJS, Walker JF, Tomkin GH, et al. Acute Wernickes encephalopathy precipitated by glucose loading. Ir J Med Sci. 1981;150(10):301-303.
22. Mayo-Smith MF, Beecher LH, Fischer TL, et al. Management of alcohol withdrawal delirium: an evidence-based guideline. Arch Intern Med. 2004;164(13):1405-1412.
23. Lexicomp Online [online database]. Hudson, OH: Wolters Kluwer Health, Inc; 2019. http://online.lexi.com. Accessed August 24, 2019.
24. Eyer F, Schuster T, Felgenhauer N, et al. Risk assessment of moderate to severe alcohol withdrawal—predictors for seizures and delirium tremens in the course of withdrawal. Alcohol Alcohol. 2011;46(4):427-433.
25. Daeppen JB, Gache P, Landry U, et al. Symptom-triggered vs fixed-schedule doses of benzodiazepine for alcohol withdrawal: a randomized treatment trial. Arch Intern Med. 2002;162(10):1117-1121.
26. Tidwell WP, Thomas TL, Pouliot JD, et al. Treatment of alcohol withdrawal syndrome: phenobarbital vs CIWA-AR protocol. Am J Crit Care. 2018;27(6):454-460.
27. Nisavic M, Nejad SH, Isenberg BM, et al. Use of phenobarbital in alcohol withdrawal management—a retrospective comparison study of phenobarbital and benzodiazepines for acute alcohol withdrawal management in general medical patients. Psychosomatics. 2019;60(5):458-467.
28. Myrick H, Anton RF. Treatment of alcohol withdrawal. Alcohol Health Res World. 1998;22(1):38-43.
29. Malcolm R, Myrick H, Brady KT, Ballenger JC. Update on anticonvulsants for the treatment of alcohol withdrawal. Am J Addict. 2001;10(s1):S16-S23.
30. Stuppaeck CH, Pycha R, Miller C, et al. Carbamazepine versus oxazepam in the treatment of alcohol withdrawal: a double-blind study. Alcohol Alcohol. 1992;27(2):153-158.
31. Hammond CJ, Niciu MJ, Drew S, Arias AJ. Anticonvulsants for the treatment of alcohol withdrawal syndrome and alcohol use disorders. CNS Drugs. 2015;29(4):293-311.
32. Wilming C, Alford M, Klaus L. Gabapentin use in acute alcohol withdrawal management. Fed Pract. 2018;35(3):40-46.
33. Stuppaeck CH, Deisenhammer EA, Kurz M, et al. The irreversible gamma-aminobutyrate transaminase inhibitor vigabatrin in the treatment of the alcohol withdrawal syndrome. Alcohol Alcohol. 1996;31(1):109-111.
34. Muzyk AJ, Fowler JA, Norwood DK, Chilipko A. Role of a2-agonists in the treatment of acute alcohol withdrawal. Ann Pharmacother. 2011;45(5):649-657.
To comment on this article, contact rdavidson@uspharmacist.com.






