US Pharm. 2019;44(1):20-23.
ABSTRACT: Alzheimer’s disease (AD) is becoming more prevalent worldwide. Four medications (donepezil, rivastigmine, galantamine, and memantine) are approved to treat AD symptoms. Despite extensive research over the past hundred years, little is known about what causes AD or how to effectively treat it. However, progress is being made in elucidating the complex pathophysiology that leads to the development of plaques and tangles, two predominant contributing factors in AD. This knowledge has fueled research aimed at developing disease-modifying agents to halt disease progression. Current research focuses on agents that target secretase enzymes to prevent plaque accumulation. Although the research appears promising, these agents have produced few successful results. The pharmacist can ensure that patients take their AD medications properly, communicate with patients and caregivers about treatment expectations, and inform them about emerging therapies.
Alzheimer’s disease (AD) is the most prevalent neurodegenerative disorder in the world. Approximately 5.5 million people in the United States and 47 million people worldwide are currently affected.1,2 Thirteen percent of people older than age 65 years and 45% of those older than age 85 years have AD, and the prevalence is increasing.3 It is expected that by 2050, one new case of AD will develop every 33 seconds, amounting to nearly a million new cases per year.4 Owing to the lack of effective treatment options, the cost of care for AD patients in the U.S.—estimated at more than $183 billion annually—is on the rise, accompanied by a corresponding increase in burden on patients’ loved ones.2 Although AD has been studied for more than a hundred years, knowledge and understanding of this complex condition are far from complete. Currently, extensive research is being conducted to determine what causes AD and to discover effective treatments.
Pathophysiology
In 1906, the neuropathologist and psychiatrist Alois Alzheimer described abnormalities found in the brain of a patient who had dementia. Although the cause of AD is still not fully understood, amyloid plaques and neurofibrillary tangles appear to be the predominant contributing factors. The production and accumulation of plaques and tangles are thought to be associated with synaptic dysfunction and neuronal degeneration that result in a slowly progressive and irreversible deterioration of memory, ultimately affecting language, personality, and cognition.2,3 Amyloid plaques are composed primarily of beta-amyloid proteins, which are derived from a parent protein called amyloid precursor protein (APP). Three secretase enzymes—alpha-, beta-, and gamma-secretase—cleave APP into soluble fragments and then clear the fragments. Amyloid plaques are believed to form when the beta- and gamma-secretase enzymes inappropriately cleave APP, leading to the creation of insoluble beta-amyloid proteins, which accumulate to form plaques in the brain; neurotoxicity and cell death result.2,3,5,6 Neurofibrillary tangles are aggregates of overphosphorylated tau proteins. Tau proteins naturally contain phosphate molecules; in AD, these proteins are hyperphosphorylated, causing the tau proteins to twist around one another and form insoluble tangles that disrupt neuronal transport. FIGURE 1 depicts the inappropriate cleaving of APP by beta- and gamma-secretase enzymes.3,4,6
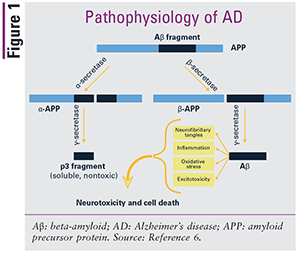
Although neuropathologists believe that plaques and tangles are the most likely cause of AD, several other possible contributing factors exist. There appears to be a genetic mechanism of AD in some families, with the e4 allele of apolipoprotein E (ApoE) conferring the strongest genetic risk identified thus far.1 One in five people carries this allele, and carriers are three times more likely than noncarriers to develop AD.1 ApoE plays a role in the processing and clearing of APP, and it is thought that carriers of the e4 allele are unable to effectively clear APP, leading to increased production and deposition of beta-amyloid. Additionally, neuron inflammation is believed to be an important factor, presenting as both a cause and a consequence of the disease. Presumably, the production of plaques and tangles is at least partly due to inflammation that occurs naturally with aging. Once formed, plaques and tangles cause more inflammation, accelerating the formation of additional plaques and tangles and leading to further cognitive decline.1,3
Although the majority of research focuses on beta-amyloid protein and tau, other risk factors for AD have been identified. Diabetes mellitus, midlife hypertension, midlife obesity, physical inactivity, depression, smoking, and low educational attainment have been identified as modifiable risk factors for the development of AD. In one statistical analysis of multiple meta-analyses, it was estimated that approximately one-third of AD cases worldwide may be related to these risk factors.7 It may be possible to prevent or delay the onset of AD by controlling these risk factors.
Current Medication Therapies
Four medications are currently available for the treatment of AD, and all were approved more than a decade ago. Of these, the first-line agents are the acetylcholinesterase (AChE) inhibitors donepezil, rivastigmine, and galantamine. These drugs increase levels of acetylcholine—an important neurotransmitter that is responsible for memory and cognitive function—in the brain by preventing enzymatic breakdown of acetylcholine. Because AChE inhibitors slow down progressive cognitive decline, they are approved to treat dementia in patients with AD.
There are no notable differences in effectiveness between these agents.8 Donepezil is commonly prescribed because it is well tolerated, but any of these agents could be used to initiate therapy. All patients should be monitored for changes in cognitive function, symptoms of gastrointestinal intolerance, and weight loss. The American Geriatrics Society’s 2015 updated Beers Criteria for Potentially Inappropriate Medication Use in Older Adults includes a strong recommendation, based on moderate-quality evidence, to avoid the use of AChE inhibitors in patients with a history of syncope because of the increased risk of bradycardia and orthostatic hypotension.9 However, practitioners must weigh specific risks versus benefits for their geriatric patients.2,9,10 See TABLE 1 for counseling points for the AChE inhibitors.
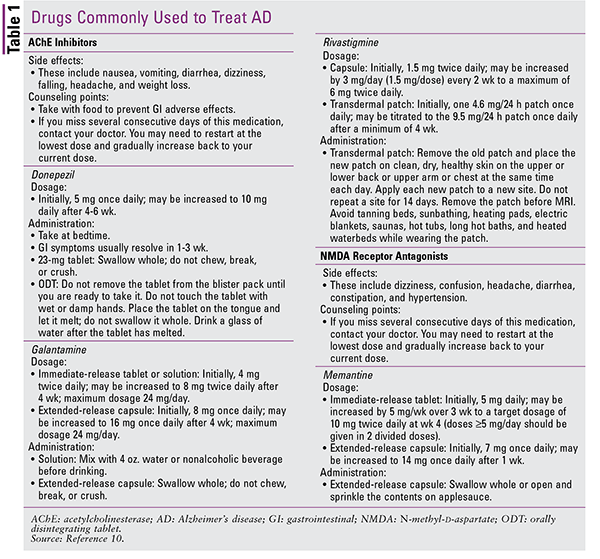
The fourth agent, memantine, is also approved for the treatment of dementia in patients with AD. Memantine is an N-methyl-d-aspartate receptor antagonist that blocks glutamate, an excitatory neurotransmitter in the central nervous system, from binding to its receptors. This prevents excessive excitotoxicity and neuronal cell death, which is thought to contribute to the pathogenesis of AD. In the late stage of AD, memantine may be combined with an AChE inhibitor, and a combination product containing donepezil and memantine is available commercially.2,6,10 Important counseling points for memantine are given in TABLE 1.
All agents used for AD slow down disease progression and may delay symptom development, but they do not significantly improve cognitive function or cure the disease. These agents are considered to be only modestly effective, and the clinical significance of their effectiveness is questionable.8 It is important for patients and family members to understand this in order to avoid unrealistic expectations.
Current Research and Possible New Agents
Increased understanding of the pathophysiology of AD has led to the development and testing of many new agents for treatment. In the AD drug-development pipeline for 2018, 112 agents were in phase I, II, or III trials; 63% of them are disease-modifying therapies (DMTs) aimed at changing the course of AD and improving outcomes rather than managing symptoms.11 About one-quarter of the drugs in development are being tested for their ability to enhance cognition, which may lead to improved memory, language, thinking, and judgment; approximately 10% of the drugs are intended to decrease behavioral symptoms such as agitation, apathy, and sleep disturbances.11,12
Most of the DMTs being studied target beta-amyloid or tau proteins.13 Inhibition of the secretase enzymes involved in generating beta-amyloid protein from APP is a primary mechanism of action for many of the drugs.11,14 Beta-site APP-cleaving enzyme (BACE) inhibitors target the beta-secretase enzymes involved in the first step of cleaving APP, whereas gamma-secretase inhibitors act on the second cleavage step.13 Many BACE inhibitors have demonstrated the ability to reduce the formation of beta-amyloid plaques, but they have not been shown to reverse existing plaques or improve cognition.13,15 Moreover, for these agents to be useful, they would have to be started early in the disease process, which is well before most AD patients are diagnosed.15 Because BACE cleaves many other critically important proteins in the brain besides APP, research must address methods to block beta-amyloid production while minimizing unwanted adverse effects.16 Gamma-secretase modulators modify the gamma-secretase enzyme to reduce beta-amyloid deposition in the brain.13,17
An emerging focus of disease-modifying therapies is the targeting of tau protein, which is associated with neurofibrillary tangles.11 Initial studies in this area, which involved reduction of tau aggregation, had disappointing results. However, these efforts raised more questions, and new strategies are being tested on seven tau immunotherapies in phase I and II trials.11
Many drugs being studied for their ability to alleviate behavioral symptoms of AD were previously approved for other diseases.12 These repurposed drugs can sometimes move from preclinical investigation to phase II clinical trials, thereby shortening an agent’s time in the drug pipeline.18 A few examples include escitalopram and mirtazapine (antidepressants), carbamazepine and levetiracetam (anticonvulsants), lithium (mood stabilizer), and methylphenidate (stimulant).12
Challenges abound in the development of effective treatment and DMTs for AD. Despite extensive research, the definitive underlying cause of this complex disease has yet to be determined. Combination therapies will likely be required, but testing strategies have focused on single-entity therapies. New therapies tested in animal models often lack predictive value in humans, and many of the drugs tested lack efficacy or have unacceptable side-effect profiles. In addition, the recruitment and retention of volunteers for lengthy drug trials is difficult, and the cost of bringing a candidate drug to market is often prohibitive. New funding strategies must be advanced in order to ensure that safe and efficacious treatments are developed to meet the urgent needs of AD patients and their loved ones.11,12,14
The Pharmacist’s Role
Because of AD’s complexity, current treatments target symptom management and merely delay disease progression. New clinical studies are changing AD therapy by shifting the focus to disease modification, but more data are necessary before these products can be brought to market. The pharmacist is in an ideal position not only to ensure that patients are taking their AD medications safely and effectively, but also to inform them about emerging therapies. As one of the most accessible healthcare providers, the pharmacist may also be involved in communicating with family members and caregivers to foster realistic treatment expectations. Given that more than a hundred agents are in the pipeline, the pharmacist’s role in the management of AD will continue to expand.
REFERENCES
1. Dos Santos Picanco LC, Ozela PF, de Fatima de Brito Brito M, et al. Alzheimer’s disease: a review from the pathophysiology to diagnosis, new perspectives for pharmacological treatment. Curr Med Chem. 2018;25(26):3141-3159.
2. Wint D. Alzheimer’s disease. www.clevelandclinicmeded.com/medicalpubs/diseasemanagement/neurology/alzheimers-disease. Accessed September 15, 2018.
3. Agamanolis DP. Neuropathology, chapter 9: Alzheimer’s disease. http://neuropathology-web.org/chapter9/chapter9bAD.html. Accessed September 16, 2018.
4. Kumar A, Singh A, Ekavali. A review on Alzheimer’s disease pathophysiology and its management: an update. Pharmacol Rep. 2015;67(2):195-203.
5. Swerdlow RH. Pathogenesis of Alzheimer’s disease. Clin Interv Aging. 2007;2(3):347-359.
6. Massoud F, Gauthier S. Update on the pharmacological treatment of Alzheimer’s disease. Curr Neuropharmacol. 2010;8(1):69-80.
7. Norton S, Matthews FE, Barnes DE, et al. Potential for primary prevention of Alzheimer’s disease: an analysis of population-based data. Lancet Neurol. 2014;13(8):788-794.
8. Winslow BT, Onysko MK, Stob CM, Hazlewood KA. Treatment of Alzheimer disease. Am Fam Physician. 2011;83(12):1403-1412.
9. American Geriatrics Society 2015 Updated Beers Criteria Expert Panel. American Geriatrics Society 2015 updated Beers Criteria for Potentially Inappropriate Medication Use in Older Adults. J Am Geriatr Soc. 2015;63(11):2227-2246.
10. Lexicomp Online [online database]. Hudson, OH: Wolters Kluwer Health, Inc; 2018. http://online.lexi.com. Accessed September 15, 2018.
11. Cummings J, Lee G, Ritter A, Zhong K. Alzheimer’s disease drug development pipeline: 2018. Alzheimers Dement (N Y). 2018;4:195-214.
12. Ellison JM. What’s new in the Alzheimer’s treatment pipeline? BrightFocus Foundation. www.brightfocus.org/alzheimers/article/whats-new-alzheimers-treatment-pipeline. Accessed October 5, 2018.
13. Scheltens P, Blennow K, Breteler MM, et al. Alzheimer’s disease. Lancet. 2016;388:505-517.
14. Cummings J, Lee G, Mortsdorf T, et al. Alzheimer’s disease drug development pipeline: 2017. Alzheimers Dement (N Y). 2017;3(3):367-384.
15. Moussa CEH. Beta-secretase inhibitors in phase I and phase II clinical trials for Alzheimer’s disease. Expert Opin Investig Drugs. 2017;26(10):1131-1136.
16. Coimbra JRM, Marques DFF, Baptista SJ, et al. Highlights in BACE1 inhibitors for Alzheimer’s disease treatment. Front Chem. 2018;6:178.
17. Kumar D, Ganeshpurkar A, Kumar D, et al. Secretase inhibitors for the treatment of Alzheimer’s disease: long road ahead. Eur J Med Chem. 2018;148:436-452.
18. Clinical trials: your questions answered. Clarksburg, MD: BrightFocus Foundation; 2018.
To comment on this article, contact rdavidson@uspharmacist.com.





