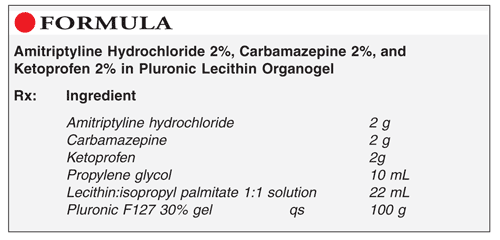
Method of Preparation: Calculate the quantity of each ingredient for the amount to be prepared. Accurately weigh or measure each ingredient. Mix the first three powders until uniformly blended. Add the propylene glycol; mix to form a smooth paste. Add the lecithin:isopropyl palmitate solution; mix well. Add sufficient Pluronic F127 30% gel to final weight; mix using a sheer mixing method. Package and label.
Use: This transdermal gel has been used in the treatment of mild-to-moderate neuropathic pain.
Packaging: Package in tight, light-resistant containers.
Labeling: Shake well before using. Keep out of the reach of children. Discard after 30 days.
Stability: A beyond-use date of 30 days may be used for this preparation.1
Quality Control: Quality-control assessment can include theoretical weight compared with actual weight, specific gravity (SG), active drug assay, color, texture-surface, texture-spatula spread, appearance, feel, rheologic properties, and physical observations.2
Discussion: Amitriptyline hydrochloride (C20H23N.HCl, MW 313.86, Elavil) is a tricyclic antidepressant (TCA) that shares the pharmacologic actions, uses, and potential toxicity of the TCAs. It occurs as a white or practically white, odorless or practically odorless, crystalline powder or small crystals. It is freely soluble in water, alcohol, chloroform, and methanol. It melts between 195˚C and 199˚C. The pH of a 1-in-100 solution is between 5.0 and 6.0.1,3,4
Carbamazepine (C15H12N2O, MW 236.27, Tegretol) occurs as a white to off-white powder that is practically insoluble in water and soluble in alcohol. Carbamazepine is an iminostilbene derivative used as an anticonvulsant, for the relief of pain associated with trigeminal neuralgia, and for some psychiatric disorders.1
Ketoprofen (C16H14O3, MW 254.28) occurs as a white or almost white, odorless or almost odorless, crystalline powder. It is practically insoluble in water, but is freely soluble in alcohol and ether. Ketoprofen has analgesic, anti-inflammatory, and antipyretic properties and is a cyclo-oxygenase inhibitor. It should be preserved in tight containers.1,5
Propylene glycol (C3H8O2) occurs as a clear, colorless, viscous, practically odorless liquid with a sweet taste, somewhat resembling glycerin. It has an SG of 1.038 g/mL and is miscible with acetone, 95% ethanol, glycerin, and water.6
Lecithin (egg lecithin, soybean lecithin, vegetable lecithin) describes a complex mixture of acetone-insoluble phosphatides--consisting chiefly of phosphatidylcholine, phosphatidylethanolamine, phosphatidylserine, and phosphatidylinositol--in combination with triglycerides, fatty acids, and carbohydrates. Physically, the products range from viscous semiliquids to powders. They are practically insoluble in water, polar solvents, and cold vegetable and animal oils; when mixed with water, however, they hydrate to form emulsions. They should be stored in well-closed containers and protected from light.7
Isopropyl palmitate (C19H38O2, MW 298.51) is a colorless, mobile liquid with a faint odor that has good spreading characteristics and is used as an emollient, oleaginous vehicle, and solvent. It is soluble in acetone, castor oil, cottonseed oil, alcohol, and mineral oil and is insoluble in water, glycerin, and propylene glycol. It should be stored in well-closed containers and protected from light.8
Pluronic F127 is a poloxamer. The poloxamers are a series of closely related block copolymers of ethylene oxide and propylene oxide that are used as emulsifying agents, solubilizing agents, and wetting agents. They are available in different grades, as either liquids or solids, with average molecular weights ranging from 2,090 to 14,600. Poloxamers generally are white-colored, waxy, free-flowing granules or cast solids that are practically odorless and tasteless. The pH of a 2.5% w/v aqueous solution is in the range of 6.0 to 7.4. Poloxamer 407 (Pluronic F127) is generally available in powder form. It is odorless or may have a very mild odor. It is freely soluble in water, alcohol, and isopropyl alcohol.9
REFERENCES
1. USP Pharmacists' Pharmacopeia. 2nd ed. Rockville, MD: U.S. Pharmacopeial Convention, Inc; 2008:68,105,218,1423,1428.
2. Allen LV Jr. Standard operating procedure for performing physical quality assessment of ointments/creams/gels. IJPC. 1998;2:308-309.
3. McEvoy GK. AHFS Drug Information 2010. Bethesda, MD: American Society of Health Systems Pharmacists; 2010:2413-2415.
4. Sweetman SC, ed. Martindale: The Complete Drug Reference. 36th ed. London, England: Pharmaceutical Press; 2009:376-382.
5. Reynolds JEF, ed. Martindale: The Extra Pharmacopoeia. 30th ed. London, England: Pharmaceutical Press; 1993:21-22.
6. Weller PJ. Propylene glycol. In: Rowe RC, Sheskey PJ, Quinn ME, eds. Handbook of Pharmaceutical Excipients. 6th ed. London, England: Pharmaceutical Press; 2009:592-594.
7. Sheng JJ. Lecithin. In: Rowe RC, Sheskey PJ, Quinn ME, eds. Handbook of Pharmaceutical Excipients. 6th ed. London, England: Pharmaceutical Press; 2009:385-387.
8. Taylor AK. Isopropyl palmitate. In: Rowe RC, Sheskey PJ, Quinn ME, eds. Handbook of Pharmaceutical Excipients. 6th ed. London, England: Pharmaceutical Press; 2009:350-351.
9. Collett JH. Poloxamer. In: Rowe RC, Sheskey PJ, Quinn ME, eds. Handbook of Pharmaceutical Excipients. 6th ed. London, England: Pharmaceutical Press; 2009:506-509.
To comment on this article, contact rdavidson@uspharmacist.com.





