US Pharm. 2022;47(9):41-46.
ABSTRACT: The global health crisis of coronavirus disease 2019 (COVID-19) continues to challenge the standard of care in both the inpatient and outpatient setting. One area that has been heavily impacted is effective management of anticoagulation. COVID-19 has led to new and innovative ways to manage vitamin K–antagonist therapy in the ambulatory care clinics, with increased utilization of drive-through testing sites, home international normalized ratio monitoring, and conversions to direct oral anticoagulants. The COVID-19 infection has been found to have an increased risk in venous thromboembolism events due to its complex inflammatory response. Since 2019, multiple strategies have been trialed in the inpatient setting, including therapeutic, intermediate, and prophylaxis anticoagulation using heparin or low-molecular-weight heparin. Vaccines and therapeutics for COVID-19 have added complexity to the clinical situation, including an FDA warning for a vaccine-induced immune thrombocytopenia with the adenoviral vector vaccine (Ad26.COV2.S) and the potential for drug interactions between COVID-19 therapeutics and anticoagulation therapy.
The global pandemic of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) led to isolation and social distancing, ultimately affecting patient access to medical services. Pharmacists continue to work with patients in the inpatient and outpatient setting and are the most accessed front-line healthcare professional. Responding to COVID-19 has led to many new and expanded roles and creative modes of pharmacy delivery, as well as adjustments to well-established clinical roles, including anticoagulation management. This infectious disease and the public health response have provided new challenges and opportunities in the pharmacist-guided management of safe and effective anticoagulation.
COVID-19 Pandemic’s Impact on Anticoagulation Management
COVID-19 has had a dramatic impact on outpatient anticoagulation management, especially with the frequent laboratory testing and follow-up required with vitamin K–antagonist (VKA) therapy. Time spent in therapeutic international normalized ratio (INR) range has been significantly lower across studies.1,2 More anticoagulant bleeds have occurred during the pandemic, and more patients have delayed seeking medical attention for VKA-associated bleeding.1,2 In a study where patients were surveyed about nonadherence to warfarin INR monitoring, 94.1% blamed the pandemic.2
Nonadherence to INR testing was most dramatic early on. In one study, from March 2020 to May 2020, INR testing volumes decreased significantly at many prothrombin time (PT)/INR testing sites during initial lockdowns. This rebounded in June 2020 and went back up to previous testing volumes. In this study, abnormally high INR (>3.5) results significantly increased in 10 out of 11 study sites. The authors recommended mitigating risk of adverse bleeding by considering the utilization of drive-through testing sites or shifting towards more home INR monitoring.3 Successful implementation of pharmacist-implemented drive-through INR testing at various locations increased patient access and mitigated health.4
A teaching hospital in London, UK, researched use of INR point-of-care (POC) testing devices on time in therapeutic range and missed appointments. Not only did INR POC testing provide convenience, but a statistically significant mean time in therapeutic range also increased from 52% to 60.7%, and there was a 39% reduction in missed appointments. Cost effectiveness and technical requirements are a concern, but positive impacts on patient outcomes and patient satisfaction warrants implementation if possible.5
Conversion to a direct-acting oral anticoagulant (DOAC) is a potential strategy to avoid nonadherence to follow-up for VKA monitoring. One population-based cohort study in England, on behalf of the National Health Service, examined whether there was higher risk of negative COVID-19 outcomes in patients on warfarin (92,339 warfarin users) compared with DOACs (280,407 users).6 Interestingly, despite obvious challenges with VKAs, patients on warfarin diagnosed with COVID-19 did as well or better compared with their matched counterparts on DOACs for COVID-19–related outcomes. In this broad data set, warfarin therapy, compared with DOAC therapy, was associated with less:
• Testing positive for COVID-19, hazard ratio (HR), 0.74 (95% CI, 0.68-0.79)
• COVID-19–related hospital admission, HR, 0.75 (95% CI, 0.68-0.83)
• COVID-19–related death, HR, 0.74 (95% CI, 0.66-0.83).
It is difficult to determine if warfarin, compared with DOACs, is protective against these COVID-19 outcomes, or if other factors biased the warfarin group toward favorable outcomes. As an observational study, the data should be interpreted cautiously, but they do provide reassurance that patients well-maintained on warfarin can continue therapy so long as continued monitoring is possible.
VTE Risks in Hospitalized COVID-19 Patients
It has been found that SARS-CoV-2 induces a complex inflammatory response that includes initiating the coagulation cascade related to von Willebrand factor, factor VII release, factor V upregulation, and platelet activation.7,8 Specifically, COVID-19 has been associated with increased inflammation and a prothrombotic state, leading to increased fibrin degradation products and increased thrombin.9 Upon admission to the hospital, fibrinogen, PT, INR, and D-dimer levels were elevated in a vast majority of patients.
These markers are associated with worse clinical outcomes, including more severe respiratory illness, venous thromboembolisms (VTEs), and death.10-12 Among the many known risk factors for mortality in patients hospitalized with COVID-19, the greatest risk is in those with an elevated D-dimer level.11 A recent cohort study found that patients remain at increased risk of pulmonary embolism 110 days after COVID-19. The same study found that patients were at a 25- to 54-fold increased risk depending on the wave of the pandemic.13 It has been demonstrated in multiple studies that mortality is higher in patients with COVID-19 who have a thromboembolism, with some studies reporting a 60% increase compared with patients without a VTE.14
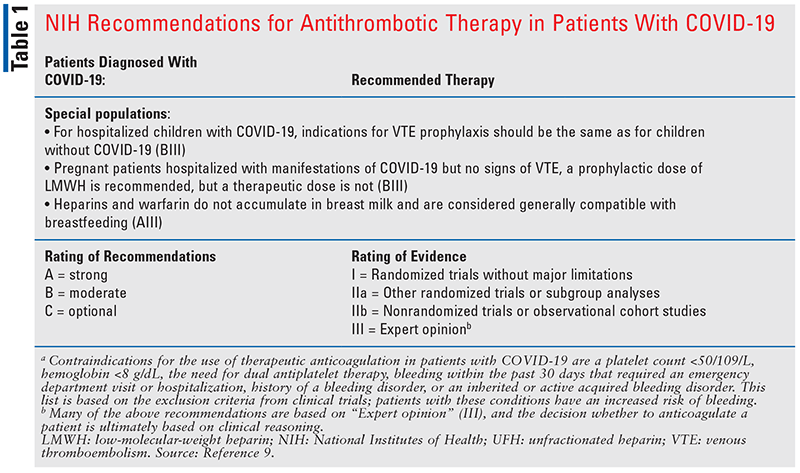
Recommendations and the standard of practice to prevent and treat VTEs have varied and evolved over time. During the wave of the Alpha variant, autopsy reports were finding microemboli and thrombosis in the lungs, heart, kidney, and liver.15 This led to the question of using prophylaxis, intermediate, or therapeutic anticoagulation doses of heparin or low-molecular-weight heparin (LMWH). Current evidence to support the use of therapeutic dosing versus prophylaxis dosing of anticoagulation is mixed, and much is based on expert opinion and extrapolation.9 One study found that in noncritically ill patients, therapeutic dosing of anticoagulants was associated with reduced use of ICU-level organ support compared with prophylaxis dosing (adjusted odds ratio [OR], 1.27; 95% CI, 1.03-1.58) and improved survival at 28 days (adjusted OR, 1.30; 95% CI, 1.05-1.61). The same study found no benefit in morbidity, mortality, or thrombotic events for therapeutic dosing of anticoagulation in critically ill patients.16 Another study found that in patients with an elevated D-dimer level greater than four times the upper limit of normal, therapeutic anticoagulation was associated with a reduced risk of VTE events and mortality compared with prophylactic or intermediate dosing of LMWH or unfractionated heparin (UFH; risk ratio [RR], 0.68; 95% CI, 0.49-0.96).17 The National Institutes of Health (NIH) 2022 guidelines (see TABLE 1) specifically address the importance of anticoagulation in patients with COVID-19, recommending full-dose anticoagulation in noncritically ill patients with an elevated D-dimer level, requiring low-flow oxygen, and who are a low bleed risk. If the patient is considered critically ill, prophylaxis anticoagulation is recommended.9
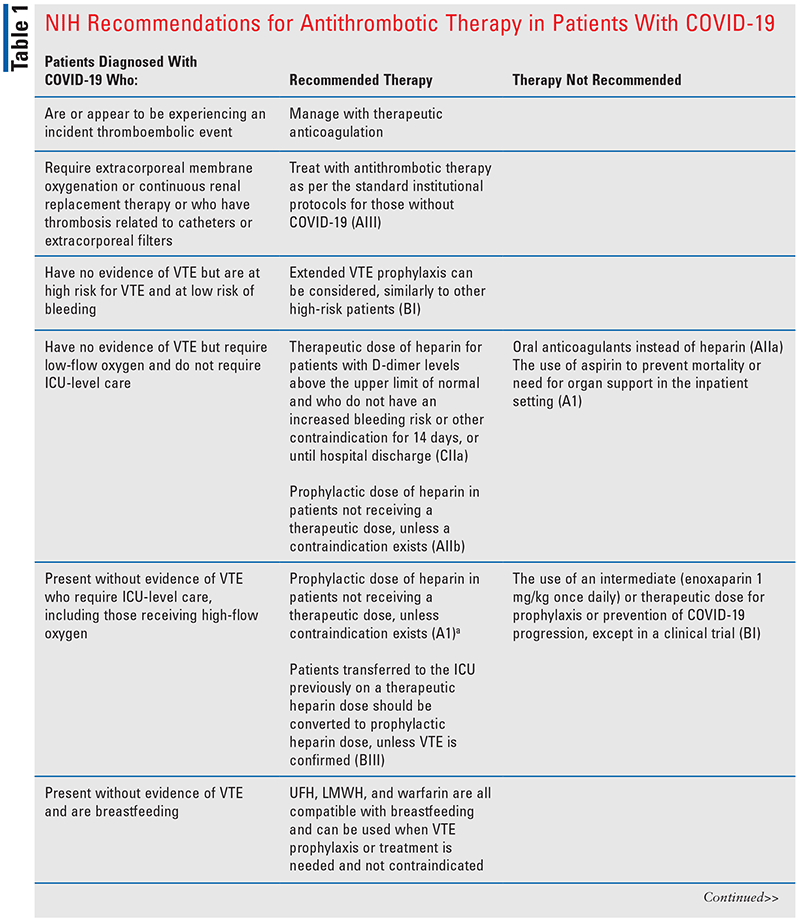
Various case reports and case series describe reduced warfarin dosing requirements in patients hospitalized with COVID-19. INR monitoring should be intensified if warfarin is continued in any setting in patients diagnosed with COVID-19. In the hospital, most patients are converted to LMWH therapy.18 Insufficient data exist in support of DOACs for the prevention of COVID-19 VTEs in the hospital setting.
Additional studies are needed to investigate the ultimate duration of anticoagulation to prevent a VTE in COVID-19 patients. In one trial, patients were randomized to continue rivaroxaban 10 mg daily or no anticoagulation for 35 days post hospital discharge. The primary outcome was a composite of VTE events and cardiovascular death; the study found a reduction in events in the rivaroxaban group (RR, 0.33; 95% CI, 0.13-0.90).19 Further studies are needed to support this finding prior to recommending routine anticoagulation on discharge.
VTE Risk and COVID-19 Infection in the Outpatient Setting
There is a strong correlation between severe COVID-19 and incidence of VTE and pulmonary embolism (42% and 17%, respectively); less data are available on the risk of VTE events in nonhospitalized patients and/or patients with less severe disease.20 One study evaluated the incidence of VTE events within 30 days of COVID-19 infection. The study found no difference in VTE events between COVID-19–infected symptomatic patients and patients with a negative result (1.8 vs. 2.2 cases per 1,000 tested; P = .16).21 Current guidelines do not recommend routine VTE prophylaxis with anticoagulation for nonhospitalized patients with COVID-19. Although no current therapy is recommended, all patients should be monitored for clinical signs and symptoms of a VTE including lower extremity pain, swelling, and edema as well as shortness of breath, hemoptysis, pleuritic pain, and new-onset cough. Additionally, nonpharmacologic measures such as ambulating, staying hydrated, and leg elevation are recommended for all patients with COVID-19, if feasible.12
Antiplatelet Drug Used to Prevent Arterial Thrombi
COVID-19 is also associated with arterial thrombus formation. For patients not requiring anticoagulation, aspirin can be considered, but data are conflicting. The NIH guidelines do not generally recommend adding antiplatelet therapy in COVID-19, especially in patients already receiving or about to receive anticoagulation, but patients already receiving antiplatelet therapies for underlying conditions should continue use.9 One meta-analysis detailed six quality studies that demonstrated benefit from aspirin initiated in the hospital, commonly around a 50% reduction. This same meta-analysis highlighted 10 quality studies that showed no benefit from aspirin on maintaining organ function, and some showed risk for mechanical ventilation. Most studies included in the analysis were retrospective and some poorly controlled.22
Anticoagulation and Vaccination Against COVID-19
By the end of May 2021, vaccination against COVID-19 was estimated to have prevented/delayed about 140,000 U.S. deaths.23 Unfortunately, vaccination has also led to unanticipated destabilization of VKA therapy in some patients, and adenoviral vector vaccination has been associated with a risk for vaccine-induced immune thrombotic thrombocytopenia (VITT).24-26
Vaccine-Induced Immune Thrombotic Thrombocytopenia
While severe COVID-19 infection is associated with many detrimental thrombotic changes, the COVID-19 vaccination has also been associated with coagulopathies and a thrombotic thrombocytopenia. Recently, the adenoviral vector vaccine (Ad26.COV2.S), which is under Emergency Use Authorization in the U.S., received strong FDA warnings regarding its risk for causing thrombotic thrombocytopenia.24 The most common site of thrombosis in VITT is the cerebral sinus vein thrombosis; thrombosis has also been reported in the splanchnic/portal veins, arterial veins, and lower extremities. Researchers estimate an incidence rate of VITT of 3.83 cases per million doses administered. This estimation is specific to VITT (not inclusive of other VTE), and the authors identify underreporting of this adverse effect as a concern.25 Women of reproductive age have been the most affected by this rare but severe adverse effect. Although the mechanism of VITT is unknown, it can resemble heparin-induced thrombocytopenia (HIT) with the formation of anti–PF4-heparin antibodies and a platelet count less than 150,000 cells/mm3. The peak onset for VITT is 6 to 14 days following vaccination. For those who suffer VITT—given its similarity to HIT—it is recommended these patients be managed with nonheparin-based anticoagulants (e.g., argatroban and bivalirudin). Intravenous immunoglobulin (1 g/kg for 2 days) to decrease antibody-induced platelet activation is also recommended by some experts for individuals with VITT.26
COVID-19 Vaccination and Destabilization of VKA Therapy
Interestingly, the BNT162b2 vaccine (Comirnaty) has been noted to quickly destabilize patients on VKAs, and the second dose is worse than the first. In standard-intensity VKA therapy (goal INR 2.0-3.0), there was an increased occurrence of both supratherapeutic INRs (OR, 1.34; 95% CI, 1.08-1.67) and subtherapeutic INRs after first vaccination (OR, 1.40; 95% CI, 1.08-1.83). The propensity for a supratherapeutic INR was even more profound in the high-intensity anticoagulation group (INR 2.5-3.5) for both the first dose (OR, 2.29; 95% CI, 1.22-4.28) and the second dose (OR, 3.25; 95% CI, 1.06-9.97). The authors advise their readers of the importance of monitoring INR after vaccination, even in stable patients.26
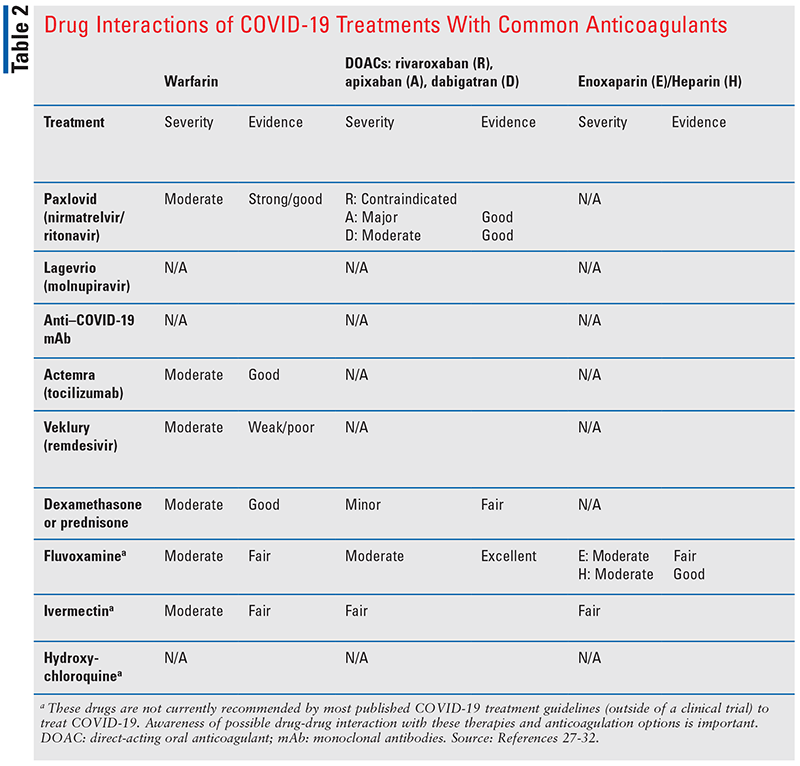
For patients on anticoagulants who develop COVID-19, the potential for drug interactions with any therapies that may be used should also be considered (see TABLE 2).
The NIH guidelines on antithrombotic therapy in patients with COVID-19 indicate that patients diagnosed with COVID-19 currently receiving anticoagulant or antiplatelet therapies for underlying conditions should continue current therapy. In patients with no evidence of VTE who are not at a high risk for VTE, anticoagulation is not recommended by the guidelines. When anticoagulants are indicated in the hospitalized patient, heparins are preferred over oral anticoagulants due to faster onset, shorter half-lives, reversibility, routes of administration, and fewer drug-drug interactions (AIII). Generally, LMWH is preferred over UFH.9 Additional recommendations are found in TABLE 1.
Conclusion
COVID-19 continues to impact the management of anticoagulation in both the inpatient and outpatient settings. Pharmacists are uniquely situated to provide anticoagulation management and evaluate and mitigate VTE risk. Prescribers of anticoagulants must be well versed on the effect of COVID-19 infection on thrombotic and inflammatory markers and increased PT/INR in patients with severe COVID-19, as well as the potential drug-drug interactions of COVID-19 therapeutics. While pharmacist-provided COVID-19 vaccination likely helped prevent many deaths and VTE events in 2021, it is not without risk in the anticoagulated population or in patients at risk for VTE. Pharmacists play an important role in the prevention and treatment of VTE events in various settings and in helping patients navigate the difficult realm of COVID-19 treatment and prevention which may impact current anticoagulation therapy.
REFERENCES
1. Emren ZY, Senöz O, Erseçgin A, Emren SV. Evaluation of bleeding rate and time in therapeutic range in patients using warfarin before and during the COVID-19 pandemic. Clin Appl Thromb Hemost. 2021;27:10760296211021495.
2. Tolga D, Fatih L. The short-term effect of the COVID-19 pandemic on the management of warfarin therapy. Kardiologiia. 2021;61(7):55-59.
3. Pearson LN, Johnson SA, Greene DN, et al. Side-effects of COVID-19 on patient care: an INR story. J Appl Lab Med. 2021;6(4):953-961.
4. Peduzzi B, Gaske Hill M, Hamilton J, et al. Drive-through point-of-care INR testing: novel concepts for delivery of care during the COVID-19 pandemic. Am J Health Syst Pharm. 2022;79(1):e4-e7.
5. Gee E, Pol A, Kittoe K, et al. Keeping warfarin patients safe during the COVID-19 pandemic: review of an INR self-testing programme. Br J Nurs. 2022;31(3):142-146.
6. Wong AYS, Tomlinson LA, Brown JP, et al. Association between warfarin and COVID-19−related outcomes compared with direct oral anticoagulants: population-based cohort study. J Hematol Oncol. 2021;14:172.
7. Stefely JA, Christensen BB, Gogakos T, et al. Marked factor V activity elevation in severe COVID-19 is associated with venous thromboembolism. Am J Hematol. 2020;95:1522-1530.
8. Ortega-Paz L, Capodanno D, Montalescot G, Angiolillo DJ. Coronavirus disease 2019−associated thrombosis and coagulopathy: review of the pathophysiological characteristics and implications for antithrombotic management. J Am Heart Assoc. 2021;10(3):e019650.
9. National Institutes of Health. Coronavirus disease 2019 (COVID-19) treatment guidelines: antithrombotic therapy in patients with COVID-19. May 31, 2022. www.covid19treatmentguidelines.nih.gov/therapies/antithrombotic-therapy/. Accessed July 25, 2022.
10. Wu T, Zuo Z, Yang D, et al. Venous thromboembolic events in patients with COVID-19: a systematic review and meta-analysis. Age Ageing. 2021;50(2):284-293.
11. Dessie ZG, Zewotir T. Mortality-related risk factors of COVID19: a systematic review and meta-analysis of 42 studies and 423,117 patients. BMC Infect Dis. 2021;21(1):855.
12. Kurtzman L. Why COVID-19 could be causing blood clots—and what you can do to lower your risk. Ohio State Health & Discovery. April 18, 2022. www.health.osu.edu/health/virus-and-infection/blood-clots-covid. Accessed May 9, 2022.
13. Katsoularis I, Fonseca-Rodríguez O, Farrington P, et al. Risks of deep vein thrombosis, pulmonary embolism, and bleeding after COVID-19: nationwide self-controlled cases series and matched cohort study. BMJ. 2022;377:e069590.
14. Chen S, Zheng T, Wang S, et. al. Association between risk of venous thromboembolism and mortality in patients with COVID-19. Int J Infect Dis. 2021;108:543-549.
15. Parra-Medina R, Herrera S, Mejia J. Systematic review of microthrombi in COVID-19 autopsies. Acta Haematol. 2021;144(5):476-483.
16. Lawler PR, Goligher EC, Berger JS, et. al. Therapeutic anticoagulation with heparin in noncritically ill patients with Covid-19. N Engl J Med. 2021;385(9):790-802.
17. Spyropoulos AC, Goldin M, Giannis D, et. al. Efficacy and safety of therapeutic-dose heparin vs standard prophylactic or intermediate-dose heparins for thromboprophylaxis in high-risk hospitalized patients with COVID-19: the HEP-COVID randomized clinical trial. JAMA Intern Med. 2021;181(12):1612-1620.
18. Irwin MN, Adie S, Sandison K, et al. Warfarin dose requirements in adults hospitalized with COVID-19 infection: a retrospective case series. J Pharma Pract. 2022;35(4):654-660.
19. Ramacciotti E, Barile Agati L, Calderaro D, et. al. Rivaroxaban versus no anticoagulation for post-discharge thromboprophylaxis after hospitalisation for COVID-19 (MICHELLE): an open-label, multicentre, randomised, controlled trial. Lancet. 2022;399(10319):50-59.
20. Zinellu A, Paliogiannis P, Carru C, Mangoni AA. INR and COVID-19 severity and mortality: a systematic review with meta-analysis and meta-regression. Adv Med Sci. 2021;66(2):372-380.
21. Roubinian NH, Dusendang JR, Mark DG, et al. Incidence of 30-day venous thromboembolism in adults tested for SARS-CoV-2 infection in an integrated health care system in Northern California. JAMA Intern Med. 2021;181(7):997-1000.
22. Zareef R, Diab M, Al Saleh T, et al. Aspirin in COVID-19: pros and cons. Front Pharmacol. 2022;13:849628.
23. Gupta S, Cantor J, Simon KI, et. al. Vaccinations against COVID-19 may have averted up to 140,000 deaths in the United States. Health Aff (Millwood). 2021;40(9):1465-1472.
24. FDA. Coronavirus (COVID-19) update: FDA limits use of Janssen COVID-19 vaccine to certain individuals. May 5, 2022. www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-limits-use-janssen-covid-19-vaccine-certain-individuals. Accessed May 9, 2022.
25. See I, Lale A, Marquez P, et al. Case series of thrombosis with thrombocytopenia syndrome after COVID-19 vaccination—United States, December 2020 to August 2021. Ann Intern Med. 2022;175(4):513-522.
26. Warkentin TE, Cuker A. COVID-19: vaccine-induced immune thrombotic thrombocytopenia (VITT). UpToDate. May 9, 2022. www.uptodate.com/contents/covid-19-vaccine-induced-immune-thrombotic-thrombocytopenia-vitt?search=covid-19-vaccine-induced-immune-thrombotic-thrombocytopenia. Accessed May 12, 2022.
27. Visser C, Biedermann JS, Nierman MC, et al. The immediate effect of COVID-19 vaccination on anticoagulation control in patients using vitamin K antagonists. Thromb Haemost. 2022;122(3):377-385.
28. Heparin (unfractionated). In: Lexicomp Online. Waltham, MA: Walters Kluwer. https://online.lexi.com/. Accessed August 4, 2022.
29. Enoxaparin (including biosimilars available in Canada). In: Lexicomp Online. Waltham, MA: Walters Kluwer. https://online.lexi.com/. Accessed August 4, 2022.
30. Dabigatran. In: Lexicomp Online. Waltham, MA: Walters Kluwer. https://online.lexi.com/. Accessed August 4, 2022.
31. Rivaroxaban. In: Lexicomp Online. Waltham, MA: Walters Kluwer. https://online.lexi.com/. Accessed August 4, 2022.
32. Apixaban. In: Lexicomp Online. Waltham, MA: Walters Kluwer. https://online.lexi.com/. Accessed August 4, 2022.
33. Warfarin. In: Lexicomp Online. Waltham, MA: Walters Kluwer. https://online.lexi.com/. Accessed August 1, 2022.
The content contained in this article is for informational purposes only. The content is not intended to be a substitute for professional advice. Reliance on any information provided in this article is solely at your own risk.
To comment on this article, contact rdavidson@uspharmacist.com.






