US Pharm. 2017;42(6)(Generic Drugs suppl):38-42.
ABSTRACT: Proton pump inhibitors (PPIs) are a class of antisecretory agents used to treat various gastrointestinal conditions, such as gastroesophageal reflux disease, Helicobacter pylori–related disorders, and gastric and duodenal ulcers. Although these agents are effective acid suppressants, the use of PPIs is frequently debated based on reports of new potential adverse events. Overutilization and drug-drug interactions are problems associated with PPIs. Additionally, since several PPIs are available OTC, inappropriate use of these agents is a significant concern. Pharmacists in all settings can have a profound impact on inappropriate PPI use by ensuring appropriate initiation and continuation of therapy, dosing, and treatment duration.
Proton pump inhibitors (PPIs) are a drug class commonly used for the treatment of various gastrointestinal (GI) disorders, including gastroesophageal reflux disease (GERD), Helicobacter pylori–related disorders, and gastric and duodenal ulcers. Although these antisecretory agents are the preferred treatment for numerous conditions, the class is plagued by overutilization and reports of potentially serious adverse effects and drug-drug interactions.1 Further, patient management of conditions treated with PPIs is complicated by the OTC availability of several of these agents. This article aims to highlight appropriate use and provide an evidence-based description of several new or heavily debated drawbacks of these agents.
Availability of PPIs
Six unique PPIs, five of which are available generically, are obtainable by prescription (TABLE 1).2-7 Low doses of omeprazole, lansoprazole, and a branded version of esomeprazole magnesium are available OTC. Omeprazole is also used in a coformulated product with sodium bicarbonate. This article will focus primarily on generically available, orally dosed PPIs.
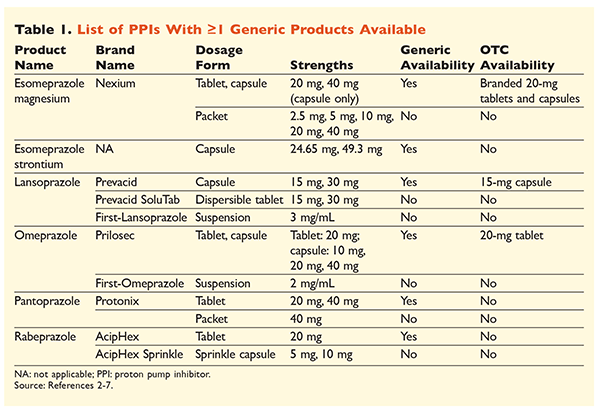
Esomeprazole strontium was approved by the FDA based on its bioequivalency to esomeprazole magnesium.3 Although the product labeling for these two agents is highly similar, three notable differences exist. First, esomeprazole strontium is available in capsules of 24.65 mg and 49.3 mg, which are equivalent to esomeprazole magnesium 20 mg and 40 mg, respectively. Second, esomeprazole strontium is not FDA-approved for use in pediatric patients, whereas esomeprazole magnesium is approved for use in those aged 1 month and older. Finally, the use of esomeprazole strontium should be avoided in severe renal impairment because it was not studied in this population. No dosage adjustment is recommended for esomeprazole magnesium.3 For the purposes of this article, the term esomeprazole will be used to describe esomeprazole magnesium.
Administration
All generically available PPIs, including capsules and tablets, should be swallowed whole without being split, crushed, or chewed.2-7 Omeprazole, lansoprazole, and esomeprazole capsules may be opened and the contents sprinkled on applesauce for patients unable to swallow oral dosage forms. Additionally, lansoprazole capsules may be sprinkled in 2 oz. of apple, tomato, or orange juice. Any mixtures containing the contents of opened capsules should be swallowed immediately and must not be stored for future use.2,4,5
Lansoprazole, omeprazole, and esomeprazole should be administered before meals, but specific recommendations on administration times vary. Omeprazole should be administered 30 to 60 minutes before a meal, and esomeprazole at least 60 minutes beforehand; a time frame is not specified for lansoprazole.2,4,5 Pantoprazole may be administered with or without food.6 Rabeprazole should be taken after a meal for duodenal ulcers and with a meal for H pylori eradication; for all other indications, rabeprazole may be taken with or without food.7
Dosing and Length of Therapy
Given the reputation of PPIs for being very effective and generally safe, significant overutilization of PPIs is a major concern.1 Not only are PPIs often administered for indications without evidence supporting their use, but these medications are also frequently prescribed at higher doses than necessary for treatment durations extending beyond recommended time frames.8,9 Each PPI’s approved dosage and recommended duration of therapy are given in TABLES 2 and 3. Treatment recommendations that differ from those approved by the FDA, as well as notable clinical pearls related to PPI initiation, dosing, and treatment duration, are outlined below.
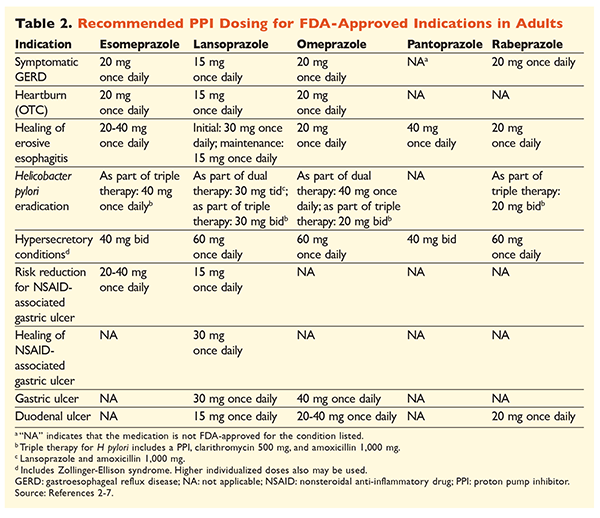
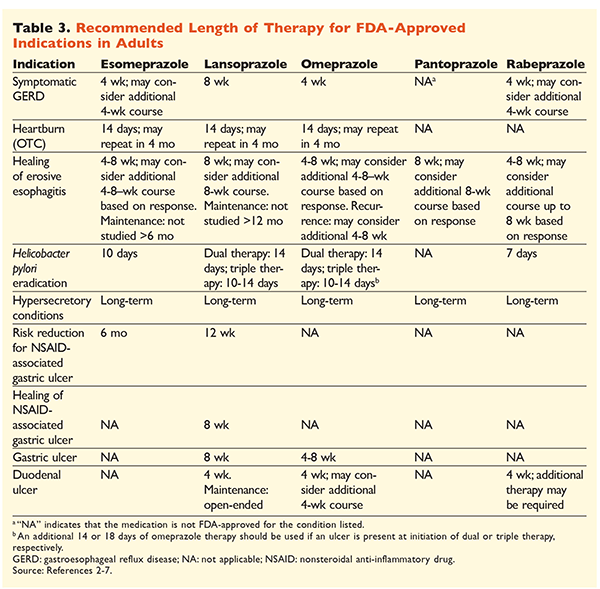
GERD: In patients with GERD, all PPIs are considered equally efficacious, and an 8-week course of therapy with any available agent is recommended.10 Patients with symptoms refractory to therapy after 2 to 3 months may require long-term maintenance therapy with twice-daily doses. However, the lowest effective dose should be used, and a different PPI should be considered if the patient does not respond to therapy.10 Alternatively, as-needed PPI therapy or a step-down approach to a histamine2 receptor antagonist (H2RA) may be considered.10 Treatment with an OTC PPI should be limited to 14 days, with retreatment occurring no more frequently than every 4 months.11
H pylori–Associated Ulcers: Depending on the PPI being used, the FDA-approved regimens for H pylori eradication include dual therapy with either amoxicillin or clarithromycin and triple therapy with both agents for 7 to 14 days.2-5,7 Specific guideline-supported treatment regimens vary compared with those approved by the FDA, but the recommended duration of therapy is similar, with all regimens having a dosing time frame of 10 to 14 days.12 After eradication of H pylori, maintenance PPI therapy is not necessary.13 A more detailed discussion of specific regimens is beyond the scope of this article.
Prophylaxis and Treatment of NSAID-Induced Ulcers: After a nonsteroidal anti-inflammatory drug (NSAID)–associated ulcer develops, the need for NSAID therapy should be reassessed. If continued NSAID therapy is deemed appropriate, patients should be maintained on selective cyclooxygenase-2 therapy at the lowest effective dose for the shortest duration possible in combination with a PPI.13
For prophylaxis, initiation of a PPI is recommended only in patients with moderate or high risk of NSAID-related GI toxicity. Risk factors include age >65 years, high-dose NSAID therapy, a previous history of uncomplicated ulcer, and concurrent use of aspirin (including low-dose), corticosteroids, or anticoagulants. Patients considered to be at moderate risk for NSAID-induced GI toxicity include those with one or two of these risk factors, whereas patients at high risk have more than two risk factors or a history of a previously complicated peptic ulcer, especially if it was recent.14 It is reasonable to continue PPI therapy as long as the moderate- or high-risk patient remains on NSAID therapy.13
Idiopathic Ulcer: Ulcers with no clearly identifiable cause are likely to recur in patients who are not treated with acid-suppressive therapy. Guidelines do not clearly recommend the length of time patients should be treated with maintenance therapy, but they suggest that ongoing PPI therapy reduces the risk of recurrent ulcer formation.13
Safety
PPIs are generally regarded as a well-tolerated class of medications.1 However, several safety concerns have long plagued their reputation, including an increased risk of Clostridium difficile–associated diarrhea, community-acquired pneumonia, and bone fractures.15,16 More recently, concerns about dementia and chronic kidney disease have been raised, and debates regarding hypomagnesemia and a theoretical drug-drug interaction with clopidogrel have continued.
Dementia: Two recent studies have suggested the potential for an increased risk of dementia with long-term PPI use in the elderly.17,18 A proposed mechanism behind this finding is that PPIs promote the development of beta-amyloid plaques, a proposed pathophysiology of Alzheimer’s disease.15 However, study limitations do not support a conclusive causal relationship between PPI use and an increased incidence of dementia.19
Renal Complications: Although PPIs have been widely recognized as a common cause of acute interstitial nephritis, newer data also implicate these agents in the development of other renal complications.15,20 Three recent trials have suggested that PPIs may be responsible for an increased risk of chronic kidney disease and progression to end-stage renal disease.20-22 However, these observational studies must be interpreted with caution, as causality cannot be confirmed.23
Hypomagnesemia: In 2011, the FDA issued a warning that prolonged use of PPIs for periods exceeding 1 year may result in hypomagnesemia.24 Since then, several sizable studies have supported this finding.25-29 The relationship between PPIs and hypomagnesemia is not definitive, however, and it is complicated by outside risk factors.30-32 Currently, clinically significant PPI-induced hypomagnesemia is viewed as rare and occurs more commonly with concomitant diuretic use.31,32 Monitoring is recommended only in patients with additional risk factors.32
Drug Interaction With Clopidogrel: Clopidogrel plays an important role in cardiovascular risk reduction, but it is associated with GI bleeds, especially in patients at increased risk. GI protection with PPIs is often implemented in patients who are more likely to experience a GI bleed, including those with a prior upper-GI bleed; advanced age; concurrent use of anticoagulants, corticosteroids, or NSAIDs (including aspirin); and/or H pylori infection.33
PPIs are thought to inhibit the CYP2C19 enzyme, the primary pathway through which clopidogrel is activated.33 This interaction can theoretically decrease the antiplatelet effects of clopidogrel and increase the risk of adverse cardiovascular outcomes.33 Based on inconsistent results from both pharmacokinetic studies and outcomes trials, however, the clinical significance of this interaction is uncertain.34
Further, there is a lack of consistency in evidence regarding the true impact of individual PPIs on the effects of clopidogrel.33 The latest FDA recommendations suggest that pantoprazole is a safer choice than other PPIs, whereas omeprazole is thought to be the riskiest.35 Current data continue to support this conclusion, but additional studies are necessary to confirm whether a difference between PPIs exists.36
It is important to weigh the benefits and risks of antiplatelet therapy alone or in combination with a PPI when a decision about adding GI-protective therapy is being made. In patients at high risk for GI complications, the beneficial effects of PPI therapy may be greater than the decreased effectiveness of antiplatelet therapy, which was inconsistently demonstrated. For patients at lower risk for GI complications, an H2RA may be a reasonable treatment option, although this class has been shown to be less effective than PPIs for this indication.33
Conclusion
Pharmacists in all settings can have a profound impact on inappropriate PPI use by ensuring proper initiation and continuation of therapy, dosing, and treatment duration. As conflicting data continue to emerge regarding the safety profiles of these medications, the long-term use of PPIs becomes more complicated. By ensuring that the lowest appropriate dose is used for the shortest duration needed, pharmacists can help prevent the unnecessary exposure of patients to potential harms.
REFERENCES
1. Heidelbaugh JJ, Kim AH, Chang R, Walker PC. Overutilization of proton-pump inhibitors: what the clinician needs to know. Therap Adv Gastroenterol. 2012;5:219-232.
2. Nexium (esomeprazole magnesium) package insert. Wilmington, DE: AstraZeneca Pharmaceuticals LP; December 2016.
3. Esomeprazole strontium package insert. Glasgow, KY: Amneal Pharmaceuticals; December 2014.
4. Prevacid (lansoprazole) package insert. Deerfield, IL: Takeda Pharmaceuticals America, Inc; October 2016.
5. Prilosec (omeprazole) package insert. Wilmington, DE: AstraZeneca Pharmaceuticals LP; December 2016.
6. Protonix (pantoprazole) package insert. Philadelphia, PA: Wyeth Pharmaceuticals Inc. October 2016.
7. AcipHex (rabeprazole) package insert. Woodcliff Lake, NJ: Eisai Inc; April 2016.
8. Chia CT, Lim WP, Vu CK. Inappropriate use of proton pump inhibitors in a local setting. Singapore Med J. 2014;55:363-366.
9. Kelly OB, Dillane C, Patchett SE, et al. The inappropriate prescription of oral proton pump inhibitors in the hospital setting: a prospective cross-sectional study. Dig Dis Sci. 2015;60:2280-2286.
10. Katz PO, Gerson LB, Vela MF. Guidelines for the diagnosis and management of gastroesophageal reflux disease. Am J Gastroenterol. 2013;108:308-328.
11. FDA Drug Safety Communication: possible increased risk of fractures of the hip, wrist, and spine with the use of proton pump inhibitors. www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm213206.htm. Accessed February 17, 2017.
12. Chey WD, Leontiadis GI, Howden CW, Moss SF. ACG clinical guideline: treatment of Helicobacter pylori infection. Am J Gastroenterol. 2017;112:212-239.
13. Laine L, Jensen DM. Management of patients with ulcer bleeding. Am J Gastroenterol. 2012;107:345-360.
14. Lanza FL, Chan FK, Quigley EM. Guidelines for prevention of NSAID-related ulcer complications. Am J Gastroenterol. 2009;104:728-738.
15. Eusebi LH, Rabitti S, Artesiani ML, et al. Proton pump inhibitors: risks of long-term use. J Gastroenterol Hepatol. 2017; Jan 16 [Epub ahead of print].
16. Abramowitz J, Thakkar P, Isa A, et al. Adverse event reporting for proton pump inhibitor therapy: an overview of systematic reviews. Otolaryngol Head Neck Surg. 2016;155:547-554.
17. Haenisch B, von Holt K, Wiese B, et al. Risk of dementia in elderly patients with the use of proton pump inhibitors. Eur Arch Psychiatry Clin Neurosci. 2015;265:419-428.
18. Gomm W, von Holt K, Thomé F, et al. Association of proton pump inhibitors with risk of dementia: a pharmacoepidemiological claims data analysis. JAMA Neurol. 2016;73:410-416.
19. Kuller LH. Do proton pump inhibitors increase the risk of dementia? JAMA Neurol. 2016;73:379-381.
20. Arora P, Gupta A, Golzy M, et al. Proton pump inhibitors are associated with increased risk of development of chronic kidney disease. BMC Nephrol. 2016;17:112.
21. Xie Y, Bowe B, Li T, et al. Proton pump inhibitors and risk of incident CKD and progression to ESRD. J Am Soc Nephrol. 2016;27:3153-3163.
22. Lazarus B, Chen Y, Wilson FP, et al. Proton pump inhibitor use and the risk of chronic kidney disease. JAMA Intern Med. 2016;176:238-246.
23. Kia L, Kahrilas PJ. Therapy: risks associated with chronic PPI use—signal or noise? Nat Rev Gastroenterol Hepatol. 2016;13(5):253-254.
24. FDA Drug Safety Communication: low magnesium levels can be associated with long-term use of proton pump inhibitor drugs (PPIs). www.fda.gov/Drugs/DrugSafety/ucm245011.htm. Accessed February 17, 2017.
25. Kieboom BC, Kiefte-de Jong JC, Eijgelsheim M, et al. Proton pump inhibitors and hypomagnesemia in the general population: a population-based cohort study. Am J Kidney Dis. 2015;66:775-782.
26. Park CH, Kim EH, Roh YH, et al. The association between the use of proton pump inhibitors and the risk of hypomagnesemia: a systematic review and meta-analysis. PLoS One. 2014;9:e112558.
27. Luk CP, Parsons R, Lee YP, Hughes JD. Proton pump inhibitor-associated hypomagnesemia: what do FDA data tell us? Ann Pharmacother. 2013;47:773-780.
28. Cheungpasitporn W, Thongprayoon C, Kittanamongkolchai W, et al. Proton pump inhibitors linked to hypomagnesemia: a systematic review and meta-analysis of observational studies. Ren Fail. 2015;37:1237-1241.
29. Markovits N, Loebstein R, Halkin H, et al. The association of proton pump inhibitors and hypomagnesemia in the community setting. J Clin Pharmacol. 2014;54:889-895.
30. Park SH, Lee SH, Lee JS, et al. Changes in serum magnesium concentration after use of a proton pump inhibitor in patients undergoing percutaneous coronary intervention. Kidney Res Clin Pract. 2015;34:98-102.
31. Danziger J, William JH, Scott DJ, et al. Proton-pump inhibitor use is associated with low serum magnesium concentrations. Kidney Int. 2013;83:692-699.
32. Begley J, Smith T, Barnett K, et al. Proton pump inhibitor associated hypomagnesaemia—a cause for concern? Br J Clin Pharmacol. 2016;81:753-758.
33. Abraham NS, Hlatky MA, Antman EM, et al. ACCF/ACG/AHA 2010 expert consensus document on the concomitant use of proton pump inhibitors and thienopyridines: a focused update of the ACCF/ACG/AHA 2008 expert consensus document on reducing the gastrointestinal risks of antiplatelet therapy and NSAID use. Am J Gastroenterol. 2010;105:2533-2549.
34. Melloni C, Washam JB, Jones WS, et al. Conflicting results between randomized trials and observational studies on the impact of proton pump inhibitors on cardiovascular events when coadministered with dual antiplatelet therapy: systematic review. Circ Cardiovasc Qual Outcomes. 2015;8:47-55.
35. FDA reminder to avoid concomitant use of Plavix (clopidogrel) and omeprazole. www.fda.gov/Drugs/DrugSafety/ucm231161.htm. Accessed February 17, 2017.
36. Bundhun PK, Teeluck AR, Bhurtu A, Huang WQ. Is the concomitant use of clopidogrel and proton pump inhibitors still associated with increased adverse cardiovascular outcomes following coronary angioplasty?: a systematic review and meta-analysis of recently published studies (2012-2016). BMC Cardiovasc Disord. 2017;17:3.
To comment on this article, contact rdavidson@uspharmacist.com.






