US Pharm. 2018;43(9):30-33.
ABSTRACT: Bacterial vaginosis (BV), a common infection in women, occurs when the balance of normal vaginal flora is disrupted by the replacement of Lactobacillus species (spp.) in the vagina with high concentrations of anaerobic bacteria. Guidelines for treating sexually transmitted diseases, which were updated by the CDC in 2015, recommend oral metronidazole, intravaginal metronidazole gel, or intravaginal clindamycin cream for the treatment of BV. Alternative agents include oral tinidazole, oral clindamycin, and clindamycin ovules. Following publication of the CDC guidelines, secnidazole—a nitroimidazole agent with a long half-life—was approved for treatment of BV. Pharmacists are in a key position to actively work with healthcare providers to select the most appropriate treatment regimen, monitor for adverse effects, and provide counseling and recommendations for preventive measures in patients with BV.
Bacterial vaginosis (BV) occurs when the balance of normal vaginal flora is disrupted.1 Because BV is not a CDC-reportable disease, the true incidence is unknown.2 According to the CDC, BV is the most common vaginal infection in females aged 15 to 44 years, and it is estimated to occur in more than 21 million of those aged 14 to 49 years.1,3 The CDC updated its guidelines for the treatment of sexually transmitted diseases, including recommendations for the management of BV, in 2015.4 This article reviews these guidelines and also provides information on secnidazole, which was approved in September 2017 for the treatment of BV.5
Etiology and Pathophysiology
BV, which is characterized by a dramatic change in the vaginal microflora, occurs when Lactobacillus species (spp.) in the vagina are replaced with high concentrations of anaerobic bacteria.2,4,6 Lactobacilli that are normally present in the vagina help maintain an acidic pH by producing lactic acid from glycogen.2,7 This acidic environment inhibits the growth of other bacteria normally found at low levels in the vagina. The decrease in commensal lactobacilli (particularly Lactobacillus crispatus, which may produce more lactic acid than other Lactobacillus spp.) is associated with increased vaginal pH and overgrowth of facultative anaerobes, including Gardnerella vaginalis, Atopobium vaginae, Mycoplasma hominis, Mobiluncus spp., and Ureaplasma spp.2,4,8,9 Additionally, certain Lactobacillus spp. produce bacteriocins (bactericidal proteins) and hydrogen peroxide, which inhibit the growth of bacteria and viruses—including HIV—in vitro.2,10
Patients with BV have a dense biofilm—consisting of a matrix of polysaccharides, proteins, extracellular DNA, and embedded bacteria—that is tightly attached to the vaginal epithelial surface.11,12 The biofilm is polymicrobial and consists mainly of G vaginalis, with 80% of G vaginalis–containing biofilms also containing A vaginae.11 Bacteria residing within a biofilm enjoy relative protection from antimicrobial agents and the host immune system, as well as high resistance to lactobacilli-produced defense factors (such as lactic acid, hydrogen peroxide, and bacteriocins), which may be due in part to differences in gene expression.10,13-15 Therefore, infections involving biofilm, such as BV, are difficult to clear and often have high rates of recurrence.13,16
Transmission and Risk Factors
It is unknown whether one pathogen transmitted through sexual exposure causes BV, and the mechanism that causes the disruption of lactobacilli that leads to BV is not completely understood.4 Sexual exposure (particularly having multiple or new sex partners) increases the risk of BV, and this condition rarely occurs in women who have never had sex. Additionally, regular douching and the absence of vaginal lactobacilli or protection during sex are related to the development of BV.4 African American and Mexican-American women have a higher prevalence of BV compared with white women, and among women who have sex with women, there is an increased likelihood that BV is present in both partners.2,3,17 Women who use estrogen-containing contraception have a decreased prevalence and rate of recurrence of BV; this may be due to increased glycogen (which is used by Lactobacillus spp. to produce lactic acid) in epithelial cells.17-19 Abstaining from sex, consistent and correct condom use, limiting the number of sexual partners, and avoiding douching are strategies that can reduce a person’s risk of BV.1,2
Diagnosis
The laboratory method that is considered the gold standard for BV diagnosis is the Gram stain. The Gram stain, which is used to identify types of bacteria, will reveal the relative concentration of the normal flora of Lactobacillus (which is a gram-positive rod) spp. compared with other types of gram-negative and gram-variable bacteria that typically overgrow in patients with BV. A normal Gram stain will show only lactobacilli or a majority of lactobacilli, whereas a Gram stain that shows more mixed flora and an absence or low quantity of lactobacilli is consistent with BV. Nugent scoring, which is based on the number of different types of microorganisms found on Gram stain, is used to determine whether a patient has BV.2,4,20
For diagnosis based on clinical symptoms, the Amsel criteria may be used. With this method, the patient must have three of the following: a thin, milky-white, homogeneous discharge that adheres to the vaginal walls; vaginal fluid pH greater than 4.5; a positive whiff test (detection of a fishy odor in vaginal discharge before or after the addition of 10% potassium hydroxide); or the presence of clue cells (characterized by bacterial clumping on the borders of vaginal epithelial cells) on wet mount microscopic examination.2,4
There are additional laboratory tests that, according to the CDC guidelines, have acceptable performance characteristics compared with Gram staining. These include a DNA probe for G vaginalis (BD Affirm VPIII) and a test (OSOM BVBLUE) that detects sialidase (an enzyme produced by bacterial pathogens associated with BV) activity in vaginal fluid. All women with BV should also be tested for HIV and other sexually transmitted infections.4
Clinical Presentation and Complications
It is estimated that one-half of women with BV are asymptomatic. In patients with symptoms, BV is characterized by a malodorous (fishy) vaginal discharge that more commonly occurs following sexual intercourse and after completion of menses.2 These symptoms may result in significantly reduced self-esteem, sexual activity, and quality of life.21 Women with BV also carry an increased risk of acquisition and transmission of sexually transmitted infections, including chlamydia, gonorrhea, herpes simplex virus type 2, and HIV.22-25 Additionally, BV is associated with preterm delivery, low infant birthweight, spontaneous abortion, postpartum endometritis, and pelvic inflammatory disease.1,2,8,17
Treatment
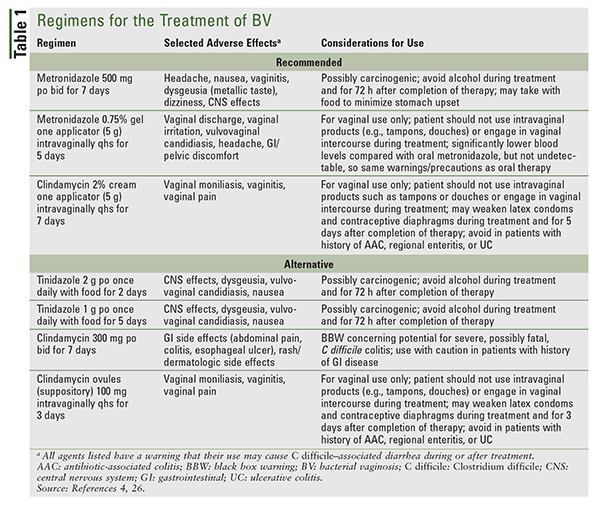
It is recommended that all women with symptomatic BV be treated.4 Benefits of therapy include relief from signs and symptoms of infection and a reduced risk of acquiring a sexually transmitted infection. An additional benefit of treatment in pregnant women with symptomatic BV is the possibility of preventing adverse pregnancy outcomes.2,4 Recommended and alternative regimens for BV treatment from the CDC’s 2015 sexually transmitted disease treatment guidelines are given in TABLE 1.4 The guidelines state that the recommended regimens may be used in both pregnant and nonpregnant women (TABLE 1). Regimens for treatment of BV that are not in the CDC guidelines include metronidazole 750 mg extended-release tablets once daily on an empty stomach for 7 days and metronidazole 1.3% gel one applicator intravaginally as a single dose.26 For patients with an allergy or intolerance to a nitroimidazole (i.e., metronidazole, tinidazole), intravaginal clindamycin cream is preferred. Patients should be counseled to avoid sexual activity, particularly if using a vaginal product, or to use condoms consistently and correctly while receiving treatment (TABLE 1). To reduce the possibility of a disulfiram-like reaction, patients should also be counseled to avoid alcohol use during and after treatment with the nitroimidazoles (TABLE 1).4
Secnidazole, a nitroimidazole agent with a long half-life, was approved in September 2017 as a single-dose oral therapy for the treatment of BV in adult women.5,27 It is supplied as a 2-g packet of granules (taken without regard to timing of meals) that should be sprinkled onto applesauce, yogurt, or pudding and entirely consumed within 30 minutes. Patients should be counseled not to chew or crunch the granules. Although secnidazole should not be dissolved in liquid, a glass of water may be taken after administration to aid in swallowing. The most common adverse reactions to secnidazole are vulvovaginal candidiasis, headache, nausea, vomiting, diarrhea, and dysgeusia. Although there were no adverse developmental outcomes in animal reproduction studies, data on the use of secnidazole in pregnant women are limited, and the drug-associated risk for developmental outcomes in humans is unknown. Patients should be counseled that alcohol use is not recommended during treatment with secnidazole and for 96 hours after administration.27,28
Because the amount of vaginal lactobacilli is reduced in patients with BV, the oral or vaginal administration of Lactobacillus spp. to replenish vaginal flora has been evaluated for BV management. The CDC’s 2015 treatment guidelines do not support the addition of Lactobacillus formulations or probiotics as adjunctive treatment or replacement therapy in BV patients, based on ongoing research on the role of these products at the time the guidelines were released.4 A recent study found that vaginally administered L crispatus (given once daily for 14 days over four menstrual cycles) decreased the rate of recurrence and prolonged the time to BV recurrence compared with placebo.29 Another recent study demonstrated that a yogurt drink containing several types of lactobacilli (strains of L crispatus, Lactobacillus gasseri, Lactobacillus jensenii, and Lactobacillus rhamnosus) that was used in combination with oral metronidazole improved the rate of recovery and reduced BV symptoms compared with placebo.7
Because the treatment of sex partners has not been shown to affect patients’ response to therapy or chance of relapse or recurrence, the routine treatment of male sexual partners of women with BV is not recommended.4,30 However, clinicians may consider screening a female sex partner of an infected woman because of the increased likelihood that BV is present in both partners.2
Follow-Up and Monitoring
The patient does not need a follow-up visit if symptoms resolve, but she should return for evaluation if symptoms occur, owing to the possibility of persistent or recurrent BV.4 Although antibiotic treatment is strongly recommended for symptomatic patients, Gardnerella spp. and other bacteria that adhere to the vaginal epithelium are protected from antibiotics by the production of biofilms, which prevent bacterial eradication.16 BV is estimated to recur in 30% of patients within 3 months after treatment of the initial infection.2 In these situations, the same recommended regimen or a different recommended regimen may be used to treat BV after the first recurrence.4 For patients with multiple recurrences following completion of a recommended regimen, a regimen of 0.75% metronidazole gel twice weekly for 4 to 6 months or an oral nitroimidazole (metronidazole or tinidazole 500 mg twice daily) for 7 days followed by intravaginal boric acid 600 mg daily for 21 days and then suppressive 0.75% metronidazole gel twice weekly for 4 to 6 months may be used.4
Conclusion
BV is common in women, and pharmacists are in a key position to counsel patients about risk factors for BV and the importance of screening. Pharmacists can actively collaborate with clinicians in the selection of the most suitable anti-infective agent for the treatment of patients with an initial or recurrent BV infection. Pharmacists should counsel patients regarding drug therapy in order to achieve optimal outcomes while minimizing side effects and unnecessary toxicity. Pharmacists should actively work with patients and other healthcare providers to ensure proper treatment and follow-up in patients with BV.
REFERENCES
1. CDC. Bacterial vaginosis—CDC fact sheet. www.cdc.gov/std/bv/BV-FS-June-2017.pdf. Accessed August 15, 2018.
2. National STD Curriculum. Vaginitis. www.std.uw.edu/go/syndrome-based/vaginal-discharge/core-concept/all. Accessed August 15, 2018.
3. CDC. Bacterial vaginosis (BV) statistics. www.cdc.gov/std/bv/stats.htm. Accessed August 15, 2018.
4. Workowski KA, Bolan GA. Sexually transmitted diseases treatment guidelines, 2015. MMWR Recomm Rep. 2015;64(RR-03):1-137.
5. FDA. Solosec (secnidazole) oral granules. www.accessdata.fda.gov/drugsatfda_docs/nda/2017/209363Orig1s000TOC.cfm. Accessed August 15, 2018.
6. Martin DH, Marrazzo JM. The vaginal microbiome: current understanding and future directions. J Infect Dis. 2016;214(suppl 1):S36-S41.
7. Laue C, Papazova E, Liesegang A, et al. Effect of a yoghurt drink containing Lactobacillus strains on bacterial vaginosis in women—a double-blind, randomised, controlled clinical pilot trial. Benef Microbes. 2018;9:35-50.
8. Bradshaw CS, Sobel JD. Current treatment of bacterial vaginosis—limitations and need for innovation. J Infect Dis. 2016;214(suppl 1):S14-S20.
9. Bai G, Gajer P, Nandy M, et al. Comparison of storage conditions for human vaginal microbiome studies. PLoS One. 2012;7:e36934.
10. Aroutcheva A, Gariti D, Simon M, et al. Defense factors of vaginal lactobacilli. Am J Obstet Gynecol. 2001;185:375-379.
11. Swidsinski A, Mendling W, Loening-Baucke V, et al. Adherent biofilms in bacterial vaginosis. Obstet Gynecol. 2005;106:1013-1023.
12. Høiby N, Ciofu O, Johansen HK, et al. The clinical impact of bacterial biofilms. Int J Oral Sci. 2011;3:55-65.
13. Machado D, Castro J, Palmeira-de-Oliveira A, et al. Bacterial vaginosis biofilms: challenges to current therapies and emerging solutions. Front Microbiol. 2016;6:1528.
14. Castro J, França A, Bradwell KR, et al. Comparative transcriptomic analysis of Gardnerella vaginalis biofilms vs. planktonic cultures using RNA-seq. NPJ Biofilms Microbiomes. 2017;3:3.
15. Patterson JL, Girerd PH, Karjane NW, Jefferson KK. Effect of biofilm phenotype on resistance of Gardnerella vaginalis to hydrogen peroxide and lactic acid. Am J Obstet Gynecol. 2007;197:170.e1-e7.
16. Swidsinski A, Mendling W, Loening-Baucke V, et al. An adherent Gardnerella vaginalis biofilm persists on the vaginal epithelium after standard therapy with oral metronidazole. Am J Obstet Gynecol. 2008;198:97.e1-e6.
17. Koumans EH, Sternberg M, Bruce C, et al. The prevalence of bacterial vaginosis in the United States, 2001-2004; associations with symptoms, sexual behaviors, and reproductive health. Sex Transm Dis. 2007;34:864-869.
18. Bradshaw CS, Vodstrcil LA, Hocking JS, et al. Recurrence of bacterial vaginosis is significantly associated with posttreatment sexual activities and hormonal contraceptive use. Clin Infect Dis. 2013;56:777-786.
19. Brooks JP, Edwards DJ, Blithe DL, et al. Effects of combined oral contraceptives, depot medroxyprogesterone acetate and the levonorgestrel-releasing intrauterine system on the vaginal microbiome. Contraception. 2017;95:405-413.
20. Bagnall P, Rizzolo D. Bacterial vaginosis: a practical review. JAAPA. 2017;30:15-21.
21. Bilardi JE, Walker S, Temple-Smith M, et al. The burden of bacterial vaginosis: women’s experience of the physical, emotional, sexual and social impact of living with recurrent bacterial vaginosis. PLoS One. 2013;8:e74378.
22. Cherpes TL, Meyn LA, Krohn MA, et al. Association between acquisition of herpes simplex virus type 2 in women and bacterial vaginosis. Clin Infect Dis. 2003;37:319-325.
23. Brotman RM, Klebanoff MA, Nansel TR, et al. Bacterial vaginosis assessed by gram stain and diminished colonization resistance to incident gonococcal, chlamydial, and trichomonal genital infection. J Infect Dis. 2010;202:1907-1915.
24. Low N, Chersich MF, Schmidlin K, et al. Intravaginal practices, bacterial vaginosis, and HIV infection in women: individual participant data meta-analysis. PLoS Med. 2011;8:e1000416.
25. Cohen CR, Lingappa JR, Baeten JM, et al. Bacterial vaginosis associated with increased risk of female-to-male HIV-1 transmission: a prospective cohort analysis among African couples. PLoS Med. 2012;9:e1001251.
26. Metronidazole. Lexicomp Online. Hudson, OH: Lexi-Comp, Inc; 2018. www.lexi.com. Accessed February 22, 2018.
27. Solosec (secnidazole) package insert. Baltimore, MD: Lupin Pharmaceuticals, Inc; October 2017.
28. Borja-Oliveira C. Alcohol-medication interactions: the acetaldehyde syndrome. J Pharmacovigilance. 2014;2:145.
29. Bohbot JM, Daraï E, Bretelle F, et al. Efficacy and safety of vaginally administered lyophilized Lactobacillus crispatus IP 174178 in the prevention of bacterial vaginosis recurrence. J Gynecol Obstet Hum Reprod. 2018;47:81-86.
30. Amaya-Guio J, Viveros-Carreño DA, Sierra-Barrios EM, et al. Antibiotic treatment for the sexual partners of women with bacterial vaginosis. Cochrane Database Syst Rev. 2016;(10):CD011701.
To comment on this article, contact rdavidson@uspharmacist.com.





