US Pharm. 2011;36(5):HS28-HS35.
Bariatric surgeries—restrictive, malabsorptive, and combination procedures—have increased in popularity since the early 1990s and are common in today’s society.1 Weight management is typically the indication for bariatric surgeries, but these procedures continue to be looked at as possible treatments for comorbidities associated with obesity, including type 2 diabetes mellitus (T2DM).1 With 8.3% of the U.S. population currently diagnosed with either type 1 or type 2 diabetes and annual costs exceeding $174 billion, new therapies and treatment approaches are continuously being researched.2 Many studies have examined the effects of bariatric surgery on T2DM, and most have shown promising results, including remission (normalization of glucose and glycated hemoglobin A1C [A1C] without need for medications).3-6 Throughout this review, the types of bariatric surgery will be discussed, along with their proposed mechanisms for inducing remission of T2DM, their effects on drug absorption, morbidity, and mortality, and their overall cost-effectiveness.
Restrictive Procedures
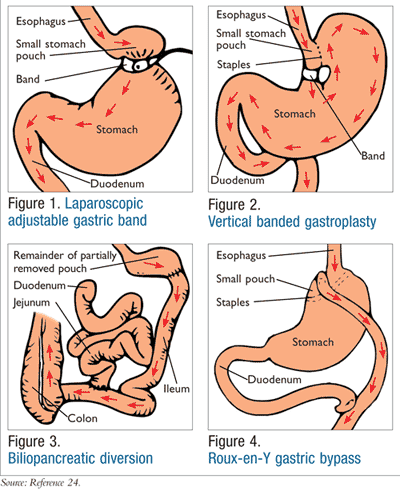
Laparoscopic adjustable gastric band surgery (LAGBS) (FIGURE 1), which is the most common restrictive procedure performed, is adjustable and reversible.7,8 In this procedure, a band is placed around the upper portion of the stomach, creating a pouch that can only hold small amounts of food, resulting in early satiety.7 LAGBS leads to significant weight loss (50%-60%); however, in most cases only 14% of the weight loss is maintained after 10 years.8,9 Vertical banded gastroplasty (VBG) (FIGURE 2) is also a restrictive procedure where, in addition to band placement, staples are used to create the pouch. Approximately 16% of weight loss is maintained over 10 years after VBG, but this surgery is not as common as LAGBS.8,9
Induction of weight loss is the primary mechanism behind resolution of T2DM when restrictive procedures are performed.1 In a randomized, controlled trial of 60 patients aged 40 to 60 years with an average body mass index (BMI) of 37 kg/m2, remission of diabetes (fasting blood glucose [BG] <126 mg/dL and A1C of <6.2%, without use of hypoglycemic medications) was achieved in 73% of the patients randomized to receive LAGBS and in only 13% of those receiving conventional therapies (i.e., medications, education) at 2-year follow-up (P <.001).3 At baseline, 28 of the surgical patients and 26 patients randomized to the conventional group were receiving hypoglycemic medication. At 2-year follow-up, only 4 patients who had undergone LAGBS were still taking hypoglycemic medications, whereas 22 of the patients from the conventional group still required therapy.3
Ponce et al completed a retrospective study of 402 patients with T2DM requiring either oral hypoglycemic medications or insulin and hypertension requiring antihypertensive therapy, who had undergone LAGBS.4 The authors determined that resolution rates (normal A1C and requiring no diabetic medications) among T2DM patients were 66%, 70.6%, and 80% at 12, 18, and 24 months, respectively. Though an overall decrease in A1C was seen at every follow-up interval, only 35% of patients with >5 years’ duration of T2DM ended up in the low A1C (£6%) group compared to 91% of patients with a disease duration <5 years (P <.001). The same relation regarding duration of T2DM was seen when looking at percentage of weight lost at 1 year (32.3% vs. 42.5%, P = .033) and 18 months (36.2% vs. 59%, P = .014) postsurgery.4
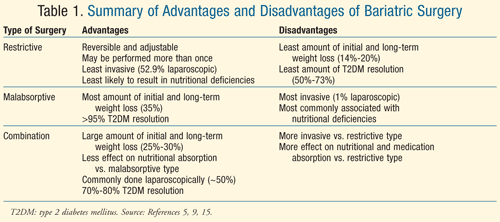
Restrictive procedures have advantages over other surgeries since nutritional deficiencies and decreased medication absorption do not occur to the extent that they do in other procedures. Additionally, the procedure is adjustable and reversible, is less invasive, and requires less time (approximately 1 hour), so the risk of infection and other complications may be smaller than with malabsorptive and combination surgeries.8 Although there are advantages, the weight loss seen with LAGBS is not as dramatic as with other forms of bariatric surgery and, as previously mentioned, is not well maintained (TABLE 1).8,9
Malabsorptive Procedures
Most malabsorptive procedures, such as biliopancreatic diversion with duodenal switch (BPD/DS) (FIGURE 3), typically lead to nutritional deficiencies, so they are not common practice. However, they result in the highest percentage of weight loss (62%-75%) and potentially lead to the highest resolution rates of T2DM.7,8 This procedure includes the removal of approximately 70% of the patient’s stomach and attachment of the duodenum to the lower portion of the small intestine, thus bypassing the jejunum. This procedure can take over 4 hours when performed laparoscopically, but when the abdominal wall is too thick, a full open procedure must be done.10
A systematic review and meta-analysis of 621 studies and over 135,000 patients with an average BMI of 47.9 kg/m2 was conducted from January 1990 to April 2006.5 Approximately 22.3% of the overall population had T2DM at the time of surgical intervention, and 10.5% had previously undergone bariatric surgery. The authors demonstrated that BPD/DS resulted in the most overall short-term and long-term (>2 years) resolution (off hypoglycemic medication, fasting BG <100 mg/dL, and A1C <6%) of T2DM (95.1%, 94%, and 95.9%, respectively) compared to LAGBS (56.7%, 55%, and 58.3%), and gastric bypass (combination procedure) (80.3%, 81.6%, and 70.9%).5
Malabsorptive procedures may result in resolution of T2DM, but there are disadvantages associated with this type of procedure as well. In a study of 312 patients with T2DM undergoing BPD at the University of Genoa School of Medicine in Italy, four deaths were attributed to nutritional deficiencies, a common complication of these types of procedures.11 A summary of advantages and disadvantages is provided in TABLE 1.
Combination Procedures
Combination procedures that consist of both restrictive and malabsorptive features include the Roux-en-Y gastric bypass (RGB) (FIGURE 4). This surgery is the most frequently performed since it results in a high percentage of weight loss initially and long-term and is commonly associated with resolution of T2DM.8,12,13 In RGB, a small pouch is formed from the stomach using staples, which is then connected to the proximal end of the jejunum, essentially skipping the duodenum.7,8 The distal end of the duodenum is then attached alongside the distal end of the jejunum, forming a Y.7 This procedure takes approximately 4 hours in the operating room, and the length of hospital stay is usually 3 to 5 days.8,14
Though weight reduction is part of the mechanism leading to remission of T2DM in patients after RGB and BPD/DS, it is not the sole process. Normoglycemia is seen much sooner in patients undergoing RGB or BPD/DS than LAGBS.7,12 There are two predominant hypotheses regarding remission of T2DM post RGB and BPD/DS. First, the “hindgut hypothesis” suggests that removing part of the gut allows glucagon-like peptide-1 (GLP-1), peptide YY, and ghrelin, among other substances, to reach the distal aspects of the intestines quicker and enhances hormonal effects.9,13,15 GLP-1 leads to increased insulin secretion and beta-cell proliferation, slows gastric emptying, and results in early satiety, which is why it has become a popular treatment in T2DM. Satiety may also be increased due to the enhanced effects of protein YY. At this time, the exact mechanism of ghrelin is not fully understood regarding long-term weight reduction.9,13,15
The second hypothesis is referred to as the “foregut hypothesis,” and it is not as well understood. This hypothesis infers that certain insulin resistance–promoting signals are omitted due to the bypassing of the duodenum and part of the jejunum.13
A prospective, nonrandomized, observational study of 10 morbidly obese patients (5 patients with T2DM and 5 without) observed an 80% (4/5) resolution (euglycemic with no need for hypoglycemic medications) rate of T2DM and saw remission (decreased need for hypoglycemic medications) in the fifth patient.16 The authors indicate that the increased concentrations of GLP-1 seen contributed to the resolution/remission of T2DM. Despite increases in GLP-1 levels, the changes in concentration seen pre- and postoperatively did not reach statistical significance.16
Combination procedures offer advantages over other bariatric surgeries, including higher rates of T2DM resolution compared to restrictive procedures and fewer nutritional deficiencies versus malabsorptive surgeries (TABLE 1).
Complications and Cost
Though the results of these studies continuously demonstrate the enormous effect bariatric surgery can have on T2DM, there are issues that need to be considered before bariatric surgery can become common practice. Many of the studies looking at bariatric surgery and T2DM are not randomized, controlled trials, so there are limitations to the data currently available. In most studies conducted, patients had a BMI >40 kg/m2, which is currently the criterion for consideration for bariatric surgery according to the National Institutes of Health (NIH) and the American Diabetes Association (ADA).17,18 Obese patients with a BMI of <35 kg/m2 have not been studied with regard to bariatric surgery and fall below the NIH and ADA cutoff of BMI >40 kg/m2 or >35 kg/m2 with the presence of one or more obesity-associated disease states.17,18 In addition, there are risks associated with these procedures, and heavy costs to both the patients and their insurance companies.17
Complications of bariatric surgery are many, and they range from minor infections to death, though the latter is rare.10 Common complications include access-site infections and ulcerations.19,20 More invasive procedures may result in more infections, but it has also been documented that patients with T2DM are more likely to experience this type of complication (P = .001), specifically when undergoing LAGBS.4 The need for repeat surgery is another potential complication and may be indicated if a hiatal hernia occurs.21
Additionally, nutritional deficiencies may result due to decreased size of the stomach and small intestines, so supplementation may be needed. Many times levels of fat soluble vitamins (A, D, E, and K), calcium, iron, vitamin B12, and folic acid are dramatically decreased due to decreased surface area for absorption. Malabsorptive surgeries typically result in the highest amount of nutritional deficiencies compared to the other forms of bariatric surgery.8 Supplementation with iron, vitamin B12, folic acid, and other vitamins and minerals should be considered in these patients.8,19 Pharmacists within all arenas should be aware of the implications of bariatric surgery with regard to nutritional deficiencies and, when possible, offer monitoring and recommendations for supplements. In many hospitals, bariatric pharmacists are collaborating with other professions to optimize patient education opportunities.
For the same reason that nutritional deficiencies occur, alterations in drug absorption are commonly seen. Agents such as metoprolol tartrate, quetiapine, and lamotrigine are rapidly and completely absorbed, indicating that alterations in the stomach and small intestines may result in alterations in their capacity for absorption.22 Since malabsorptive or combination procedures affect absorption to a greater extent than restrictive procedures, alternative formulations and routes of medication delivery should be considered (e.g., suspension, intramuscular, or IV) if these surgeries are performed. Because of drug pharmacokinetics and dynamics, extended-release formulations should not be used if possible because gastric emptying time will be affected. Additionally, medications that can lead to ulcers should be avoided in these surgical patients, or risk versus benefit should be assessed (e.g., use of aspirin, bisphosphonates).8
Another aspect to consider is decreased availability of stomach acid. Medications requiring an acidic environment, such as iron, should be given with vitamin C or other substances that enhance the acidity of the stomach.8 The continued need for hypoglycemic and antihypertensive medications, among others, should be assessed frequently since drastic weight loss may result in decreased medication need for chronic diseases.3,6
Mortality: Mortality is a very rare complication of bariatric surgery, but it is still a concern. Operative mortality for restrictive, malabsorptive, and combination procedures is 0.1%, 1.1%, and 0.5%, respectively.9 Long-term mortality for all types of procedures is slightly higher with a 0.9% 30-day mortality rate and a 6.4% 5-year mortality rate, but is still relatively low. A retrospective cohort study of >9,000 patients demonstrated that at a mean 7.1-year follow-up, surgical intervention versus no intervention for obesity resulted in a decrease of all-cause mortality by 40%, with 37.6 versus 57.1 deaths per 10,000 patients per year (P <.001).23 This study also showed a 56% decrease in death due to coronary artery disease (P = .006), and a 92% decrease in mortality due to diabetes (P = .005).23
Despite a decrease in mortality in these groups, an increase was seen when looking at mortality resulting from nondisease causes such as suicide or accidental death (11.1 vs. 6.4 per 10,000 patients per year; P = .04).23 These results indicate that behavioral health issues may require greater attention pre- and postsurgery to address the psychological and emotional issues that accompany the dramatic changes associated with bariatric surgery.24
Cost: Cost is also a factor that must be taken into consideration, since most patients cannot afford bariatric procedures on their own and, without justification, usually will not be covered by insurance. The estimated overall medical cost without insurance for bariatric surgery is over $25,000, but many studies have demonstrated that the long-term costs of T2DM care outweigh the immediate costs of bariatric surgery.25 An Australian study conducted in 2009 estimated that after 10 years, the full cost related to the surgical procedure could be recouped, though a similar study found it may only take 4 years to recover the costs.26 This study also indicated that due to the huge health care costs of conventional T2DM treatment, a total of $2,400 per patient could be saved if surgical intervention was utilized in place of conventional therapy.26 Overall, there are enormous areas of cost savings associated with surgical intervention, and these procedures extend patients’ lives (approximately 1.5 years).23
Recommendations
The NIH and ADA have published consensus statements regarding patient eligibility for bariatric surgery. As stated previously, both the NIH and the ADA recommend weight loss surgery as long as patients can demonstrate failed previous attempts at weight loss and have a BMI >40 kg/m2 or >35 kg/m2 with one or more obesity-associated disease states.17,18 Additionally, several studies have determined that patients may respond better to weight loss surgery in respect to T2DM resolution if they were recently diagnosed (i.e., within 5 years of diagnosis), though this has not been addressed by the ADA or NIH.4 Recently, the FDA’s Gastroenterology and Urology Devices Panel voted to approve the use of a particular LAGBS device in patients with a BMI >30 kg/m2 with one or more obesity-associated disease states.27 Though this less invasive procedure has been approved for utilization in patients with a lower BMI, until further data become available regarding other types of bariatric surgery and patients with a BMI <35 kg/m2, these procedures may not be recommended.
Conclusion
Though restrictions may still be in place regarding eligibility to undergo bariatric surgery, these interventions remain a promising treatment option for patients who meet the criteria. Further research will hopefully continue to demonstrate the effectiveness of bariatric surgery in the treatment of T2DM and to address other populations affected by this disease (i.e., those with BMI <35 kg/m2). Health care practitioners and patients alike must remember that lifestyle modifications are at the center of this therapy and must be continued even after surgery is completed to ensure continued success of therapy and reap the long-term benefits. Once the decision to have bariatric surgery has been made by the health care team and the patient, it is imperative for pharmacists to appropriately educate patients on this lifelong endeavor.
REFERENCES
1. Goldfine AB, Shoelson SE, Aguirre V. Expansion and contraction: treating diabetes with bariatric surgery. Nat Med. 2009;15:616-617.
2. American Diabetes Association. Diabetes statistics. www.diabetes.org/diabetes-
3. Dixon JB, O’Brien PE, Playfair J, et al. Adjustable gastric banding and conventional therapy for type 2 diabetes. JAMA. 2008;299:316-323.
4. Ponce J, Haynes B, Paynter S, et al. Effect of Lap-Band-induced weight loss on type 2 diabetes mellitus and hypertension. Obes Surg. 2004;14:1335-1342.
5. Buchwald H, Estok R, Fahrbach K, et al. Weight and type 2 diabetes after bariatric surgery: systematic review and meta-analysis. Am J Med. 2009;122:248-256.
6. Hall TC, Pellen MG, Sedan PC, et al. Preoperative factors predicting remission of type 2 diabetes mellitus after roux-en-Y gastric bypass surgery for obesity. Obes Surg. 2010;20:1245-1250.
7. Ferrannini E, Mingrone G. Impact of different bariatric surgical procedures on insulin action and beta-cell function in type 2 diabetes. Diabetes Care. 2009;32:514-520.
8. Weinberg MA, Segelnick SL. Surgical procedures for weight loss. US Pharm. 2009;34(12):HS2-HS10.
9. Vetter ML, Cardillo S, Rickels MR, et al. Narrative review: effect of bariatric surgery on type 2 diabetes mellitus. Ann Intern Med. 2009;150:94-108.
10. Søvik TT, Taha O, Aasheim ET, et al. Randomized clinical trial of laparoscopic gastric bypass versus laparoscopic duodenal switch for superobesity. Br J Surg. 2010;97:160-166.
11. Scopinaro N, Marinari GM, Camerini GB, et al. Specific effects of biliopancreatic diversion on major components of metabolic syndrome. Diabetes Care. 2005;28:2406-2411.
12. Lima MM, Pareja JC, Alegre SM, et al. Acute effect of roux-en-Y gastric bypass in whole-body insulin sensitivity: a study with the euglycemic-hyperinsulinemic clamp. J Clin Endocrinol Metab. 2010;95:3871-3875.
13. Rubino F, Forgione A, Cummings DE, et al. The mechanism of diabetes control after gastrointestinal bypass surgery reveals a role of the proximal small intestine in the pathophysiology of type 2 diabetes. Ann Surg. 2006;244:741-749.
14. Gastric bypass surgery. Mayo Foundation for Medical Education and Research. www.mayoclinic.com/health/
15. Vincent RP, le Roux CW. Changes in gut hormones after bariatric surgery. Clin Endocrinol. 2008;69:173-179.
16. Whitson BA, Leslie DB, Kellogg TA, et al. Entero-endocrine changes after gastric bypass in diabetic and nondiabetic patients: a preliminary study. J Sur Res. 2007;141:31-39.
17. NHLBI Obesity Education Initiative. Clinical Guidelines on the Identification, Evaluation, and Treatment of Overweight and Obesity in Adults. The Evidence Report. Bethesda, MD: National Heart, Lung, and Blood Institute; 1998. NIH Pub. No. 98-4083.
18. American Diabetes Association. Standards of medical care in diabetes–2011. Diabetes Care. 2011;34(suppl 1):s11-s61.
19. Nyenwe EA, Jerkins TW, Umpierrez GE, et al. Management of type 2 diabetes: evolving strategies for the treatment of patients with type 2 diabetes. Metabolism. 2011;60:1-23.
20. Sugerman HJ, Wolfe LG, Sica DA, et al. Diabetes and hypertension in severe obesity and effects of gastric bypass-induced weight loss. Ann Surg. 2003;237:751-758.
21. Mitchell MT, Carabetta JM, Shah RN, et al. Duodenal switch gastric bypass surgery for morbid obesity: imaging of postsurgical anatomy and postoperative gastrointestinal complications. Am J Roentgenol. 2009;193:1576-1580.
22. Miller AD, Smith KM. Medication and nutrient administration considerations after bariatric surgery. Am J Health Syst Pharm. 2006;63:1852-1857.
23. Adams TD, Gress RE, Smith SC, et al. Long-term mortality after gastric bypass surgery. N Engl J Med. 2007;357:753-761.
24. Weight-control Information Network. Bariatric Surgery for Severe Obesity. Bethesda, MD: National Institute of Diabetes and Digestive and Kidney Diseases; March 2009. NIH Pub. No. 08-4006. http://win.niddk.nih.gov/
25. Weight loss surgery cost and financing. Einstein Industries, Inc. www.docshop.com/education/
26. Keating CL, Dixon JB, Moodie L, et al. Cost-effectiveness of surgically induced weight loss for the management of type 2 diabetes: modeled lifetime analysis. Diabetes Care. 2009;32:567-574.
27. Walker EP. FDA panel says okay to lower BMI for Lap-Band. December 3, 2010. www.medpagetoday.com/
To comment on this article, contact rdavidson@uspharmacist.com.





