US Pharm. 2019;44(8):29-35.
ABSTRACT: In the United States, basal cell carcinoma (BCC) is diagnosed in approximately 2 million people each year. The most common cause of BCC is a mutation in the sonic hedgehog signaling pathway that is caused by ultraviolet damage and leads to uncontrolled cell growth. The hedgehog pathway inhibitors are a novel drug class initially created to treat advanced or metastatic BCC. Unique adverse effects of this class include muscle spasms, alopecia, increased creatine kinase levels, and anorexia. Patients can develop resistance to hedgehog pathway inhibitors. Clinical trials are investigating the use of hedgehog pathway inhibitors in a variety of malignancies and treatment algorithms.
Although it seldom makes headlines, basal cell carcinoma (BCC) is the most common cancer in the United States.1,2 Approximately 2 million cases of this skin cancer are reported in the U.S. each year, exceeding the incidence of all other cancers combined. Despite this large number, BCC has been difficult to study owing to its exclusion from cancer registries such as the Surveillance Epidemiology and End Results program. Additionally, the ability to study BCC was hindered until recently because the International Statistical Classification of Diseases and Related Health Problems (ICD)-9 code for BCC also included squamous cell carcinoma (SCC) of the skin.3 (The ICD-10 codes the two diseases separately.)
The prognosis for BCC is usually good; however, the incidence of this malignancy is increasing rapidly. Although it is rarely metastatic upon presentation, BCC can cause substantial destruction of local tissue, leading to disfigurement.1 Most BCCs occur on the head or neck or on sites that were exposed to radiation, whether from the sun or prior radiation therapy.4 The most common cause of BCC is a mutation in the sonic hedgehog signaling pathway that is caused by ultraviolet (UV) damage and leads to uncontrolled cell growth. The relationship between BCC and sunlight is complex and depends on timing, pattern, and amount of UV radiation exposure. Fair skin, red or blond hair, and light eye color are independently associated with BCC because of their greater susceptibility to UV damage.5,6
Another major risk factor for developing BCC is chronic immunosuppression, such as organ transplantation. Among organ-transplant patients, BCC has an incidence five- to 10-fold higher than that for the general population and can occur in up to 50% of patients in the 10 years following transplantation.7 A meta-analysis showed that in these patients BCC was more likely to occur in areas other than the head and neck and in those who were younger (a mean of 15 years younger).8 Expert panels consider these patients to have high-risk tumors.1
Genetics
As noted above, mutation in the sonic hedgehog signaling pathway—which is involved in tissue maintenance, renewal, and regeneration—plays a pivotal role in BCC pathogenesis.9,10 The PTCH1 gene on chromosome 9q codes for the sonic hedgehog receptor. When PTCH1 mutates, through UV damage or other mechanisms, the Smoothened (SMO) protein is constantly activated to stimulate downstream DNA replication and cell division. PTCH1 mutation is present in 30% to 90% of sporadic BCC cases.1,9 UV-induced mutation in the tumor-suppressor gene p53 is also common in BCC development.11
Risk Stratification
The initial workup includes a patient history and physical examination, as well as a complete skin examination to rule out any concurrent precancers or cancers. The suspicious lesion is biopsied, including the deep dermal layer. Imaging studies are performed when extensive or metastatic disease is present.12 Once the workup is completed, a risk assessment (TABLE 1) is calculated to determine the treatment plan.1
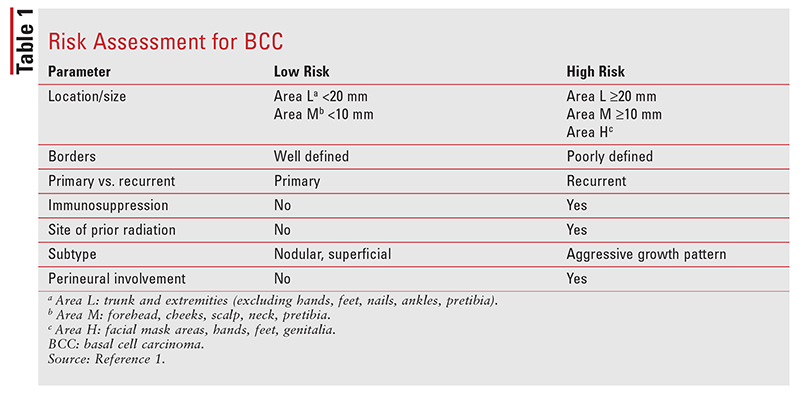
Treatment of Low-Risk Disease
Primary treatment options for low-risk disease include curettage and electrodesiccation in areas without hair growth, standard excision (if lesion can be excised with 4-mm margins), and radiation therapy (in nonsurgical candidates).1 If surgery or radiation therapy is not feasible, superficial therapies—i.e., topical 5-fluorouracil or imiquimod, photodynamic therapy, and cryotherapy—may be considered.1 Imiquimod has been shown to be effective for treating multiple superficial BCCs, with one prospective trial reporting a 5-year disease-free rate of 85%.13 The clinical success rate (absence of initial treatment failure or disease recurrence at 3 years after start of treatment) is significantly higher in patients who undergo surgical excision (P <.001). This consideration must be weighed against the cosmetic outcome, which is significantly better with imiquimod than with surgery (excellent/good at 3-year follow-up: 61% vs. 36%, respectively; P <.0001).14
High-Risk Disease
Recommended treatment options for high-risk lesions are standard excision with margins >4 mm, Mohs micrographic surgery (MMS), and radiation therapy (in nonsurgical candidates). Of these treatments, MMS is preferred because it includes intraoperative analysis of the excision margin, which has been shown to have a lower BCC-recurrence rate than standard surgical excision.15 If clear margins (i.e., no tumor cells at the outer edge of the removed tissue) cannot be achieved after primary surgery, treatment via a clinical trial or hedgehog pathway inhibitor should be considered.1
Recurrent or Metastatic Disease
The abovementioned treatments may also be used in patients with local recurrent BCC. However, if the disease has spread to the lymph nodes or a distant site, systemic treatment options, such as hedgehog pathway inhibitors, are necessary.1 Of the three currently available hedgehog pathway inhibitors, two are FDA-approved for BCC (TABLE 2).16-18
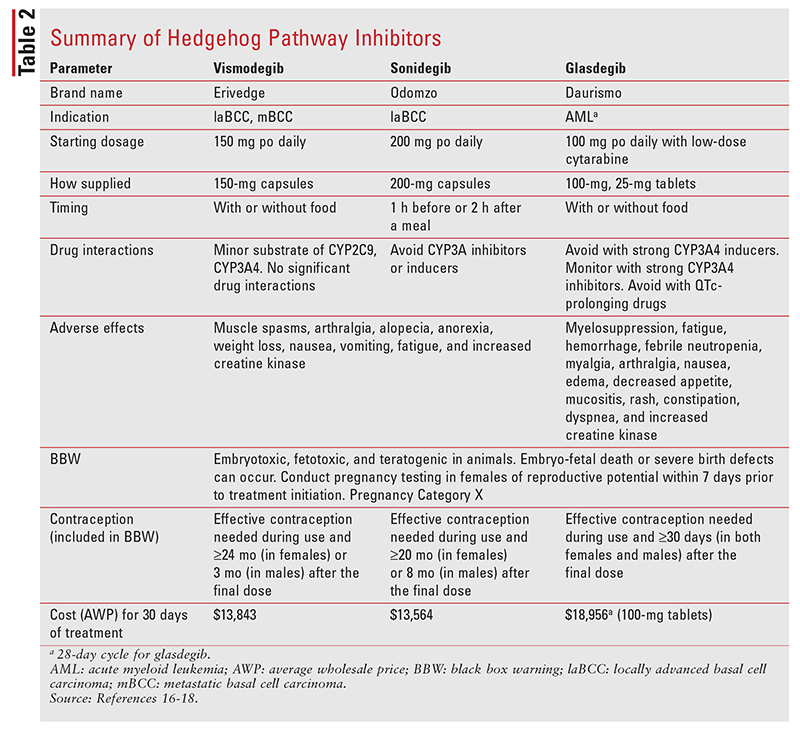
Vismodegib: In 2012, vismodegib was granted FDA approval as the first-in-class hedgehog pathway inhibitor. Approval was based on a multicenter, single-arm, two-cohort, open-label, phase II trial (ERIVANCE) involving 104 patients.19 Approximately 95% of subjects were previously treated with surgery, radiation, and/or systemic therapy. The overall response rate (ORR) in patients with locally advanced BCC (laBCC) was 43% (CI, 31-56; P <.001), and 21% had a complete response; duration of response (DOR) was 7.6 months. In patients with metastatic BCC (mBCC), the ORR was 30% (CI, 16-48; P = .001) and DOR was 7.6 months.19 A long-term response was seen in 3-year follow-up; median overall survival was not reached in patients with laBCC and was 33.4 months in patients with mBCC.20 Based on the significant results for laBCC and mBCC, the FDA approved both indications. The most common adverse effects (AEs; occurring in >20% of patients) included muscle spasms, alopecia, dysgeusia, decreased weight, fatigue, nausea, decreased appetite, and diarrhea. Median time to onset of AE was less than 6 months.19
Sonidegib: This hedgehog pathway inhibitor gained FDA approval in 2015 based on results of the phase II, randomized, double-blind, multicenter BOLT trial.21 BOLT initially compared two different doses of sonidegib (200 mg vs. 800 mg) in patients with laBCC that was not amenable to surgery or mBCC that had exhausted all other treatment options. Response rates were similar between the two doses, but the 800-mg dose was associated with a higher rate of AEs leading to treatment discontinuation (14% for 200 mg vs. 30% for 800 mg). Accordingly, the 800-mg dose was not included in 30-month follow-up, and standard dosing of 200 mg was continued.21 Unlike vismodegib, sonidegib is approved only for laBCC; this is because 30-month follow-up of sonidegib showed significant differences in ORR (56.1% vs. 7.7%), DOR (26.1 vs. 24 months), and 2-year overall survival (93.2% vs. 69.3%) in laBCC versus mBCC, respectively.22 The most common AEs (>20% of patients) included muscle spasms, alopecia, dysgeusia, diarrhea, decreased weight, increased creatine kinase (CK), fatigue, myalgia, and decreased appetite. Elevated CK level was one of the most common grade 3/4 AEs and a unique class AE for the hedgehog pathway inhibitors.21
Treatment Challenges
A key limitation to hedgehog pathway inhibitor therapy is that advanced BCC can develop resistance in the SMO protein, which limits the duration of treatment response. The ERIVANCE study estimates that 20% of patients will develop a mutation while being treated with vismodegib.20 A small investigator-initiated trial in vismodegib-resistant patients observed no response during sonidegib treatment administered for a median of 6 weeks (range, 3-58 weeks), and five of nine patients had disease progression.23
Clinical trials are being conducted to explore various dosing regimens of vismodegib and sonidegib in a variety of treatment settings, including different tumor sizes and use before or after surgery. Other hedgehog pathway inhibitors are being investigated in BCC patients to discover whether higher response rates or a more durable response can be achieved. Because of the rarity of advanced cases, the literature on systemic chemotherapy for BCC is limited to case reports.1
Follow-Up
An important fact guiding the follow-up schedule of BCC patients is that 30% to 50% of patients will develop another BCC within 5 years. This is a 10-fold increase compared with the general population.24 BCC patients are also at increased risk for developing SCC and cutaneous melanoma. Continued long-term surveillance of the patient is essential. Examination by a physician should be performed every 6 to 12 months during the first 2 years, after which the frequency may be reduced. It is also important for patients to be counseled on sun protection and regular skin self-examination.1
The Pharmacist’s Role
Hedgehog pathway inhibitors are oral chemotherapy agents that are most commonly filled through a specialty pharmacy. In this setting, the pharmacist is the healthcare professional that patients will come to with questions about AEs and management. In particular, the pharmacist should counsel the patient on potential fertility risks associated with these medications. All drugs in this class carry black box warnings for embryo-fetal toxicity, as they were found to be embryotoxic, fetotoxic, and teratogenic in animal studies (Pregnancy Category X). Effective contraception is recommended for all patients being treated with these medications.16-18
All female patients of reproductive potential should receive pregnancy testing prior to starting treatment, and they should not become pregnant or breastfeed while receiving treatment or become pregnant for up to 24 months afterward (TABLE 2). Pregnant women should not handle the medication. Male patients must use condoms—even post vasectomy—during treatment and for up to 8 months afterward (TABLE 2). Semen should not be donated for 3 months after the final dose owing to the potential to pass on teratogenic effects. Patients should not donate blood for approximately 24 months after the final dose (30 days for glasdegib).16-18
As a pharmacotherapy expert, the pharmacist is responsible for screening the patient’s profile for any drug interactions and making recommendations to the healthcare team. Owing to the high cost of these medications, the pharmacist should also facilitate insurance approval and patient enrollment in financial-assistance programs if medication coverage is denied or the copayment is too expensive.
Conclusion
BCC is the most common cancer in the U.S., but it is typically underreported to monitoring institutions because most cases are caught early and removed by excision alone. Sun protection and regular skin examinations are important patient-education points that can lead to a reduction in the rate of BCC. Up to 90% of BCC cases have a mutation in the hedgehog pathway, which led to the development of a unique drug class: the hedgehog pathway inhibitors. Owing to the rarity of laBCC and mBCC, additional studies on future treatment options are difficult to conduct. The hedgehog pathway inhibitors are currently being studied in various tumor types and different treatment algorithms. The pharmacist plays a critical role in managing AEs and medication profiles and in helping the patient with drug acquisition.
REFERENCES
1. National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines). Basal cell skin cancer. Version 1.2019. www.nccn.org/professionals/physician_gls/pdf/nmsc.pdf. Accessed May 15, 2019.
2. Miller DL, Weinstock MA. Nonmelanoma skin cancer in the United States: incidence. J Am Acad Dermatol. 1994;30:774-778.
3. Asgari MM, Moffett HH, Ray GT, Quesenberry CP. Trends in basal cell carcinoma incidence and identification of high-risk subgroups, 1998-2012. JAMA Dermatol. 2015;151:976-981.
4. Perkins JL, Liu Y, Mitby PA, et al. Nonmelanoma skin cancer in survivors of childhood and adolescent cancer: a report from the Childhood Cancer Survivor Study. J Clin Oncol. 2005;23:3733-3741.
5. Kaskel P, Lange U, Sander S, et al. Ultraviolet exposure and risk of melanoma and basal cell carcinoma in Ulm and Dresden, Germany. J Eur Acad Dermatol Venereol. 2015;29:134-142.
6. Khalesi M, Whiteman DC, Tran B, et al. A meta-analysis of pigmentary characteristics, sun sensitivity, freckling and melanocytic nevi and risk of basal cell carcinoma of the skin. Cancer Epidemiol. 2013;37:534-543.
7. Kanitakis J, Alhaj-Ibrahim L, Euvrard S, Claudy A. Basal cell carcinomas developing in solid organ transplant recipients: clinicopathologic study of 176 cases. Arch Dermatol. 2003;139:1133-1137.
8. Harwood CA, Proby CM, McGregor JM, et al. Clinicopathologic features of skin cancer in organ transplant recipients: a retrospective case-control series. J Am Acad Dermatol. 2006;54:290-300.
9. Lesiak A, Sobolewska-Sztychny D, Majak P, et al. Relation between sonic hedgehog pathway gene polymorphisms and basal cell carcinoma development in the Polish population. Arch Dermatol Res. 2016;308:39-47.
10. Xie J, Murone M, Luoh SM, et al. Activating Smoothened mutations in sporadic basal-cell carcinoma. Nature. 1998;391:90-92.
11. Ling G, Ahmadian A, Persson A, et al. PATCHED and p53 gene alterations in sporadic and hereditary basal cell cancer. Oncogene. 2001;20:7770-7778.
12. Chen J, Ruczinski I, Jorgensen TJ, et al. Nonmelanoma skin cancer and risk for subsequent malignancy. J Natl Cancer Inst. 2008;100:1215-1222.
13. Quirk C, Gebauer K, De’Ambrosis B, et al. Sustained clearance of superficial basal cell carcinomas treated with imiquimod cream 5%: results of a prospective 5-year study. Cutis. 2010;85:318-324.
14. Arits AH, Mosterd K, Essers BA, et al. Photodynamic therapy versus topical imiquimod versus topical fluorouracil for treatment of superficial basal-cell carcinoma: a single blind, non-inferiority, randomised controlled trial. Lancet Oncol. 2013;14:647-654.
15. Mosterd K, Krekels GA, Nieman FH, et al. Surgical excision versus Mohs’ micrographic surgery for primary and recurrent basal-cell carcinoma of the face: a prospective randomised controlled trial with 5-years’ follow-up. Lancet Oncol. 2008;9:1149-1156.
16. Erivedge (vismodegib) package insert. South San Francisco, CA: Genentech USA, Inc; February 2019.
17. Odomzo (sonidegib) package insert. Cranbury, NJ: Sun Pharmaceutical Industries, Inc; May 2019.
18. Daurismo (glasdegib) package insert. New York, NY: Pfizer Inc; November 2018.
19. Sekulic A, Migden MR, Oro AE, et al. Efficacy and safety of vismodegib in advanced basal-cell carcinoma. N Engl J Med. 2012;366:2171-2179.
20. Sekulic A, Migden MR, Lewis K, et al. Pivotal ERIVANCE basal cell carcinoma (BCC) study: 12-month update of efficacy and safety of vismodegib in advanced BCC. J Am Acad Dermatol. 2015;72:1021-1026.
21. Midgen MR, Guminski A, Gutzmer R, et al. Treatment with two different doses of sonidegib in patients with locally advanced or metastatic basal cell carcinoma (BOLT): a multicentre, randomised, double-blind phase 2 trial. Lancet Oncol. 2015;16:716-728.
22. Lear JT, Midgen MR, Lewis KD, et al. Long-term efficacy and safety of sonidegib in patients with locally advanced and metastatic basal cell carcinoma: 30-month analysis of the randomized phase 2 BOLT study. J Eur Acad Dermatol Venereol. 2018;32:372-381.
23. Danial C, Sarin KY, Oro AE, Chang AL. An investigator-initiated open-label trial of sonidegib in advanced basal cell carcinoma patients resistant to vismodegib. Clin Cancer Res. 2016;22:1325-1329.
24. Marcil I, Stern RS. Risk of developing a subsequent nonmelanoma skin cancer in patients with a history of nonmelanoma skin cancer: a critical review of the literature and meta-analysis. Arch Dermatol. 2000;136:1524-1530.
To comment on this article, contact rdavidson@uspharmacist.com.





