US Pharm. 2019;44(5):32-36.
The human papillomavirus (HPV), a virus belonging to the papillomavirus family and consisting of a group of more than 170 related viruses, is one of the most common sexually transmitted viruses in the world. This double-stranded DNA virus causes genital warts and premalignant and malignant lesions of the cervix, vagina, vulva, anus, penis, and oropharynx.1 HPV may infect epithelial cells of the skin or the inner lining of tissues, which are classified as either cutaneous or mucosal types. They may be further categorized into high- and low-risk groups.
Each HPV virus is given a number to specify the type. There are currently more than 100 different genotypes of HPV, of which 14 are considered high-risk for cervical cancer and its precursor lesions.2 Though most cases of cervical cancer worldwide are caused by HPV, two HPV types—16 and 18—are responsible for approximately 60% to 70% of cases.3
Epidemiology
According to the CDC, approximately 79 million Americans, primarily in their late teens to early 20s, are infected with HPV.4 Additionally, cervical cancer is recognized by the World Health Organization as the fourth largest contributor to female cancer mortality worldwide, claiming an estimated 270,000 lives annually.5
Transmission
HPV may be transmitted through vaginal, anal, or oral sex with another infected individual, even when that person is asymptomatic.6 Although occurrence is rare, transmission from mother to newborn can lead to respiratory and laryngeal papillomatosis, which may affect the newborn’s breathing tubes and voice box. Additionally, chronic complications can be the result of such transmissions.6
Diagnosis
Precancerous cervical cell changes and early cancers of the cervix generally do not cause symptoms. For this reason, regular screening using the Papanicolaou (Pap) and HPV tests are the most effective methods of diagnosis.7 Pap tests can inform a clinician if there are changes to the cervical cells.2 If those cells are abnormal, an HPV test may be subsequently performed to determine if those cervical changes are due to a high-risk strain of HPV.
Current guidelines for cervical-cancer screening recommend that women should start screening with the Pap test at age 21. Based on recommendations made by the U.S. Preventive Services Task Force, women ages 30 to 65 years should get either a Pap test alone every 3 years, cotesting with a Pap and HPV test every 5 years, or an HPV test alone every 5 years.8
Management
The CDC now recommends children aged 11 to 12 years receive two doses of the HPV vaccine to protect against cancers caused by the virus, with the second dose given 6 to 12 months after the first dose.4 The bivalent (Cervarix), quadrivalent (Gardasil), and 9-valent (Gardasil 9) vaccines collectively offer protection against HPV types 6, 11, 16, 18, 31, 33, 45, 52, and 58. Current vaccines address approximately 70% of cervical cancers through protection from HPV-16 and HPV-18.1
Once infected, there is no cure for HPV. However, treatment is directed to the macroscopic or pathologic precancerous lesions caused by HPV, such as genital warts, benign respiratory tract tumors, precancerous changes at the cervix, and cancers resulting from HPV infections.7 Methods commonly used to treat precancerous cervical changes include cryosurgery, conization, and excision. TABLE 1 further explains the various surgical procedures.9 Treatments for other types of benign respiratory tract tumors and precancerous changes caused by HPV (vaginal, vulvar, penile, and anal lesions) and genital warts include topical chemicals or medications, excisional surgery, cryosurgery, electrosurgery, and laser surgery.6
BD Onclarity HPV Assay
The Becton Dickinson (BD) Onclarity HPV Assay (FIGURE 1) specifically identifies types 16, 18, and 45, while concurrently detecting types 31, 33, 35, 39, 51, 52, 56, 58, 59, 66, and 68.10 This test requires clinicians to collect cervical specimens using the included Onclarity HPV Cervical Brush Collection Kit. Specimens collected should be placed in a BD SurePath liquid-based cytology vial.2
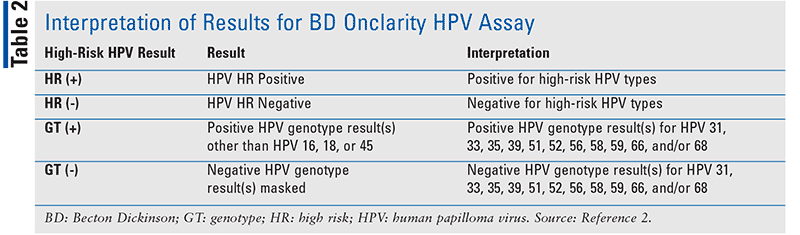
The Onclarity HPV Assay is performed with the BD Viper LT System (FIGURE 2). The assay may be used in accordance with clinical guidelines for cervical cancer screening to identify the presence of high-risk types. However, the BD Onclarity HPV Assay is not intended for women who have undergone a hysterectomy, nor has it been tested in women with prior ablative or excisional therapy or in those who are pregnant.2 Detection of high-risk HPV is dependent on the number of copies present in the specimen and may be affected by a multitude of factors, such as the stage of infection, specimen-collecting methods, and other patient-specific factors. Optimal performance of this test requires adequate specimen collection, with appropriate transport, storage, and processing; a negative test result does not exclude the possibility of infection.2 TABLE 2 indicates how best to interpret the results of the assay.
Efficacy
The BD Onclarity HPV Assay was evaluated through a multiyear, prospective, multicentered clinical trial conducted in the United States that included more than 33,500 women who either had or had not received the HPV vaccine.2 According to one study, the BD Onclarity HPV Assay was compared with the Qiagen Hybrid Capture II (HC2), an HPV DNA assay for the detection of cervical intraepithelial neoplasia (CIN), and it was found that the concordance between the BD Onclarity HPV Assay and the Qiagen HC2 Assay was 94.6%. Although the overall sensitivities for the BD Onclarity HPV Assay and the HC2 Assay were respectively 95.2% and 96.9%, the overall specificity was higher with the BD Onclarity HPV Assay (50.3%) than the HC2 Assay (40.8%).11
In addition, the BD Onclarity HPV Assay brings the added clinical information of genotyping compared with the “positive/negative” result of the HC2 Assay.11 This ability provides various benefits: First, it allows identification of the different high-risk HPV types that helps in patient-specific risk-stratification, such as reducing the burden of repeated testing against the risk of CIN3+ disease. Moreover, providing information on the HR-HPV genotypes plus the rare HPV-66 allows surveillance to be part of regular screening activity, potentially creating a cost-benefit synergy for clinical laboratories and healthcare authorities. Finally, extended genotyping enables longitudinal, genotype-specific assessments to identify persistent HPV types in women attending cervical-cancer screening programs.12 This research concluded that accurate, population-based genotyping information provided by the BD Onclarity HPV Assay has the potential to increase clinical knowledge and provide better risk stratification based on individual patients.
Conclusion
In summary, the introduction of DNA methods for identifying HPV has changed diagnosis and management dramatically. The ability to pinpoint specific genotypes has been shown to improve diagnosis and enhance development of vaccines. The BD Onclarity HPV Assay provides the benefit of being the first fully automated assay that offers extended genotyping beyond genotypes 16 and 18. In future regulatory submissions, BD intends to seek approval for reporting genotypes beyond 16, 18, and 45, which is consistent with the assay’s extended genotyping capabilities. More information can be found at www.accessdata.fda.gov/cdrh_docs/pdf16/P160037C.pdf.2
REFERENCES
1. Joura EA, Giuliano AR, Iversen OE, et al. A 9-valent HPV vaccine against infection and intraepithelial neoplasia in women. N Engl J Med. 2015;372(8):711-723.
2. FDA. Center for Devices and Radiological Health. BD Onclarity HPV Assay - P160037. www.accessdata.fda.gov/cdrh_docs/pdf16/P160037C.pdf. Accessed April 30, 2019.
3. De Sanjose S, Quint WG, Alemany L, et al. Human papillomavirus genotype attribution in invasive cervical cancer: a retrospective cross-sectional worldwide study. Lancet Oncol. 2010;
11(11):1048-1056.
4. CDC. Human papillomavirus. Published November 16, 2017. www.cdc.gov/std/hpv/stdfact-hpv.htm. Accessed November 18, 2018.
5. World Health Organization. (2015). Human papillomavirus (HPV) and cervical cancer. Fact sheet N380. www.who.int/mediacentre/factsheets/fs380/en/. Accessed April 19, 2019.
6. American Cancer Society. HPV and HPV testing. www.cancer.org/cancer/cancer-causes/infectious-agents/hpv/hpv-and-hpv-testing.html. Accessed November 18, 2018.
7. National Cervical Cancer Coalition. Cervical cancer causes, diagnosis and symptoms. www.nccc-online.org/hpvcervical-cancer/cervical-cancer-overview. Accessed November 18, 2018.
8. U.S. Preventive Services Task Force. Final update summary: cervical cancer: screening. August 2018. www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/cervical-cancer-screening. Accessed November 18, 2018.
9. CS Mott Children’s Hospital. Michigan Medicine. Cone biopsy (conization) for abnormal cervical cell changes. www.mottchildren.org/health-library/hw27835. Accessed March 11, 2019.
10. BD Onclarity™ HPV. BDJ. www.bdj.com/en-us. Accessed April 30, 2019.
11. Bottari F, Sideri M, Gulmini C, et al. Comparison of Onclarity Human Papillomavirus (HPV) assay with Hybrid Capture II HPV DNA Assay for detection of cervical intraepithelial neoplasia grade 2 and 3 lesions. J Clin Microbiol. 2015;53(7):2109-2114.
12. Ejegod DM, Serrano I, Cuschieri K, et al. Clinical validation of the BD Onclarity™ HPV Assay using a non-inferiority test. J Med Micro Diagn. Published December 28, 2013. OMICS International. www.omicsonline.org/open-access/clinical-validation-of-the-bd-onclarity-hpv-assay-using-a-noninferiority-test-2161-0703.S3-003.php?aid=22510. Accessed November 25, 2018.
To comment on this article, contact rdavidson@uspharmacist.com.