Chlamydia and gonorrhea are two of the most commonly reported bacterial sexually transmitted infections (STIs) in the United States. These STIs affect both men and women and can be associated with several long-term consequences if not treated properly. Chlamydia is often asymptomatic for both men and women, making it easily transmissible. However, untreated or incorrectly treated chlamydia infections can cause an array of complications, including cervicitis, proctitis, urethritis, epididymitis, pelvic inflammatory disease, pregnancy complications, and infertility or sterility.1,2 Pregnant women who have chlamydia at the time of delivery can also pass the infection to their baby.
Gonorrhea infections are also sometimes asymptomatic, although less commonly. Symptoms of gonorrhea in men include discolored discharge from the penis, burning upon urination, or swollen testicles. Symptoms in women include burning upon urination, increased vaginal discharge, and abnormal spotting between periods.3 Untreated gonorrhea complications include pelvic inflammatory disease in women and epididymitis in men, both of which could lead to infertility or sterility.
Both of these STIs can also increase the risk for HIV.2,3 Coinfections with gonorrhea and chlamydia are also common, due to the often asymptomatic nature of these infections.
Epidemiology
It is estimated that one in five people in the U.S. has an STI, with the most recent data showing that there were 26 million new STIs in 2018. Chlamydia and gonorrhea are two of the most common STIs, with chlamydia having a prevalence of 2.4 million and incidence of 209,000, and gonorrhea having an incidence of 4 million and a prevalence of 1.6 million. Nearly half (45.5%) of new STIs occur in teenagers and young adults, most commonly between the ages of 15 and 24 years. STIs continue to be a large financial burden for the U.S. healthcare system, with just chlamydia, gonorrhea, and syphilis accounting for over $1 billion in direct medical costs in 2018.1
Etiology and Transmission
Neisseria gonorrhoeae, a gram-negative bacterium, is the etiological agent for gonorrhea infections, and Chlamydia trachomatis, also a gram-negative bacterium, is the etiological agent for chlamydia infections.4 Both gonorrhea and chlamydia are STIs spread by having unprotected vaginal, oral, or anal sex. They can cause infections in the genital area, rectum, and throat. In women, infections can also spread to the cervix. Both of these infections can also be passed to a newborn via vaginal birth if a mother is infected at the time of delivery. Since these infections are often asymptomatic, they are easily transmissible. These infections can be prevented by abstaining from sex, being in a mutually monogamous relationship, or practicing safe sex practices, such as using a condom.
Diagnosis
There are different tests that may be utilized to diagnose chlamydia and gonorrhea. These tests include laboratory-based methods as well as point-of-care testing (POCT) that utilize nucleic acid amplification tests (NAAT) or polymerase chain reaction (PCR) methods.5 Laboratory-based testing generally requires specific transportation and collection methods, which could potentially delay diagnosis and treatment of an STI. POCT methods, which are done at or near the site of care, are generally inexpensive, are rapid, and can provide accurate results during the patient visit. The World Health Organization considers the lack of POCT for STI to be an obstacle for global STI prevention.6
Management
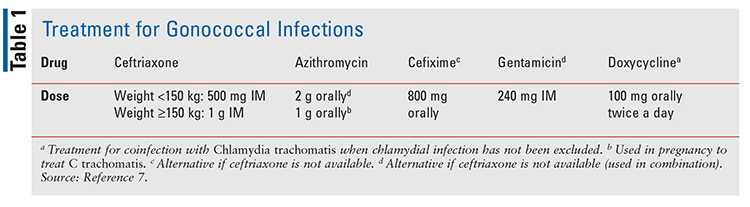
Increasing rates of antimicrobial resistance led to the revision of the CDC update to the treatment for gonococcal infections in 2020. For the treatment of uncomplicated urogenital, anorectal, and pharyngeal gonorrhea, ceftriaxone given intramuscularly is recommended. If chlamydia infection has not been excluded, concurrent treatment with doxycycline administered for 7 days is also recommended.7 For uncomplicated gonococcal infections of the pharynx, ceftriaxone is recommended, and if chlamydia coinfection exists, doxycycline is recommended for 7 days. There are no alternative treatment recommendations available for pharyngeal gonorrhea. Drug names and doses used in the treatment of chlamydia and gonorrhea are shown in TABLE 1.
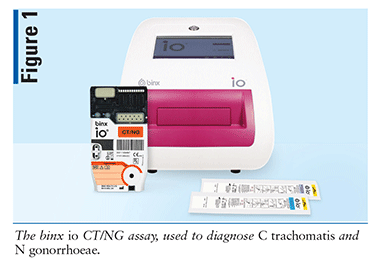
Device
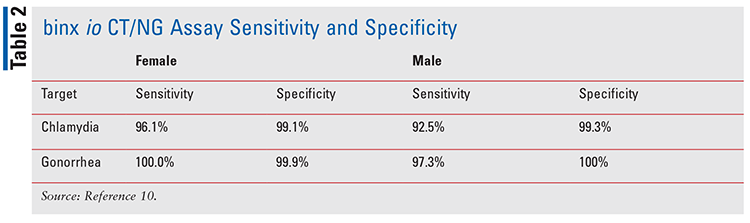
Designed by Binx Health Limited, the binx io CT/NG assay (FIGURE 1) is a clinical testing device used in the diagnosis of C trachomatis and N gonorrhoeae. In 2021, the FDA granted a clinical laboratory improvement amendment (CLIA) waiver for the binx io system to be used in physician offices, urgent care facilities, community health clinics, and retail settings with results in about 30 minutes.8 The binx io system assay uses a rapid PCR combined with a proprietary electrochemical detection to diagnose patients who have infections with chlamydia and gonorrhea.9 This device does not require manual manipulation, and the specimens may be obtained in a clinical setting or self-collected by a patient in a clinical setting. The binx io instrument processes the single-use, CT/NG cartridge that contains all reagents for use with no sample prep required. The results are easy to understand and displayed as detected or not detected. The device targets the chlamydia and gonorrhea genomic DNA. Sensitivity and specificity of the device can be found in TABLE 2.
Efficacy
Several studies have demonstrated the efficacy of POCT in the treatment of STIs. In a study published in 2020 that was conducted by Van Der Pol and colleagues, the binx io CT/NG assay was compared with three FDA-approved NAAT devices. The study enrolled 1,523 women and 922 men conducted at 11 clinics throughout the U.S. It concluded that the binx io assay was associated with good performance compared with laboratory-based molecular diagnostics for vaginal swab samples and male urine samples.10 Of note, 94.8% (2,318 of 2,445) of the tests were performed by non-laboratory personnel. In an earlier study conducted by Widdice and colleagues that compared the performance of the Atlas io diagnostic platform (binx io system) to Aptima Combo 2 and evaluated patient attitudes toward POCT by non–laboratory-trained personnel, the study concluded that the Atlas io device had higher sensitivity and specificity in women, 83.9% and 98.8%, respectively.11 Most patients were willing to wait in the clinic for results if they could be treated before leaving.
Conclusion
Although laboratory-based diagnostic options are available, the development of the binx io CT/NG assay has paved the way for early accurate diagnosis and the subsequent effective management of chlamydia and gonorrhea at the point of care. This can decrease patients lost to follow-up and improve treatment success. More information can be found at https://mybinxhealth.com/point-of-care/.
REFERENCES
1. CDC. Sexually transmitted disease at a glance, 2020. www.cdc.gov/std/statistics/prevalence-2020-at-a-glance.htm. Accessed April 5, 2021.
2. CDC. Detailed STD facts—chlamydia. www.cdc.gov/std/chlamydia/stdfact-chlamydia-detailed.htm. Accessed April 5, 2021.
3. CDC. Detailed STD facts—gonorrhea. www.cdc.gov/std/gonorrhea/stdfact-gonorrhea-detailed.htm. Accessed April 5, 2021.
4. Hill SA, Masters TL, Wachter J. Gonorrhea—an evolving disease of the new millennium. Microb Cell. 2016;3(9):371-389.
5. CDC. Recommendations for the laboratory-based detection of Chlamydia trachomatis and Neisseria gonorrhoeae—2014. MMWR Recommm Rep. 2014;63(RR-02):1-19.
6. World Health Organization. Point-of-care diagnostic tests (POCTs) for sexually transmitted infections (STIs). www.who.int/reproductivehealth/topics/rtis/pocts/en/. Accessed April 13, 2021.
7. St. Cyr S, Barbee L, Workowski KA, et al. Update to CDC’s treatment guidelines for gonococcal infection, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:1911-1916.
8. Binx Health press release. binx Health receives FDA CLIA waiver for chylamydia and gonorrhea test, expanding critical access to single-visit diagnoses. https://mybinxhealth.com/news/binx-health-receives-fda-clia-waiver-for-chlamydia-and-gonorrhea-test-expanding-critical-access-to-single-visit-diagnoses/. Accessed April 8, 2021.
9. Binx Health point-of-care testing. https://mybinxhealth.com/point-of-care. Accessed April 13, 2021.10. Van Der Pol B, Taylor SN, Mena L, et al. Evaluation of the performance of a point-of-care test for chlamydia and gonorrhea. JAMA Netw Open. 2020;3(5):e204819.
11. Widdice LE, Hsieh YH, Silver B, et al. Performance of the
Atlas rapid test for Chlamydia trachomatis and women’s attitudes toward point-of-care testing. Sex Transm Dis. 2018;45(11):723-727.
The content contained in this article is for informational purposes only. The content is not intended to be a substitute for professional advice. Reliance on any information provided in this article is solely at your own risk.
To comment on this article, contact rdavidson@uspharmacist.com.






