US Pharm. 2017;42(2)(Specialty & Oncology suppl):3-6.
ABSTRACT: Biological agents are utilized in oncology practice for cancer therapy and the supportive management of treatment-related side effects. A biosimilar is a biological product that is shown to be highly similar to a licensed biological product (the reference product) with no clinically meaningful differences from the reference product in terms of purity, safety, or potency. It is important for oncology practitioners to be knowledgeable about current biosimilars and those in development for cancer treatment in order to provide guidance and make an informed decision when incorporating these drugs into clinical practice.
Biologics for the treatment and supportive care of cancer have enhanced the therapeutic options for clinicians in the management of oncologic therapy. Monoclonal antibodies (mAbs) are employed as targeted treatment in cancer pathogenesis and in growth factors utilized to resolve therapy-related hematologic deficiencies. With the patents set to expire for a number of medications, interest related to the development of biosimilar agents has expanded for cost-saving benefits and global access.1-3
Biosimilars are defined as biological products that are shown to be highly similar to a licensed biological product (the reference product) with no clinically meaningful differences from the reference product in terms of purity, safety, or potency.4-6 The recent approval of several biosimilars in the United States has the potential to offer cost savings and health gains for patients with rheumatic diseases and cancers through highly similar efficacy.7
Biologics and generic drugs are not classified as the same. Generic drugs are small molecules that are easy to replicate, while biologics are complex molecules that are produced through living cells. Because biosimilars are produced in living cells, variation may occur by reason of posttranslational modifications such as glycosylation, which may impact drug efficacy or safety. Hence, the reason the approval process for biosimilars is so rigorous.4-6 The approval of any biosimilar is based on robust analytics and nonclinical and clinical studies depicting that the biosimilar and the reference product are highly similar with no clinically meaningful differences in relation to purity, safety, and efficacy.4-6
This article will present an overview of the regulatory requirements for approval, extrapolation of indication, emerging biosimilar mAbs, and potential limitations to the uptake of biosimilars in clinical cancer therapy.
Regulatory Requirements for Approval of Biosimilars in Oncology
Regulatory requirements for the approval of biosimilars in guidelines of the European Medicines Agency (EMA), the FDA, and the World Health Organization (WHO) are science-based and similar.4-6 The approval of “biosimilarity” is based on the comparison of the proposed biosimilar to the reference product with respect to structure, function, animal toxicity, human pharmacokinetics and pharmacodynamics, clinical immunogenicity, clinical safety, and efficacy. Overall, biosimilarity is confirmed when “the biological product is highly similar to the reference product notwithstanding minor differences in clinically inactive components. Furthermore, there are no clinically meaningful differences between the biological product and the reference product in terms of the safety, purity, and potency of the product.”5
The objective of a biosimilar development program is not to reestablish benefit but rather to demonstrate that there are no clinically meaningful differences based on the robust evidence. A stepwise process is conducted that begins with an analytical and nonclinical comparison of structural and in vitro functional characteristics followed by nonclinical in vivo animal studies and clinical studies.4-6 The set and amount of data that are considered to be sufficient to demonstrate biosimilarity are determined on a product-specific basis.
The structural and in vitro functional characteristics are the foundation of biosimilar development and consist of the analysis of primary, secondary, tertiary, and quaternary structures that includes aggregation, post-translational modification (i.e., glycosylation, phosphorylation, and deamidation), intentional chemical modification (i.e., pegylation), and biologic activity.5 The necessary extent of the nonclinical in vivo animal studies and clinical studies is dependent on the evidence from the preceding step.4-6
Final approval is based on one or more comparative clinical studies within a suitable clinical setting with at least one study that assesses immunogenicity, pharmacokinetics, or pharmacodynamics that demonstrate safety, purity, and clinical efficacy of the biosimilar.8 For example, in the case of Filgrastim Hexal in Europe, the comparison of efficacy to the reference product filgrastim (Neupogen) was based on a pharmacodynamics study in healthy volunteers that was considered acceptable by the EMA.9 In the case of filgrastim-sndz (Zarxio) in the U.S., the comparison of efficacy to the reference product Neupogen was considered acceptable by the FDA.10
Overall, during the evaluation of the biosimilarity of a proposed biosimilar compared to the reference product, regulatory agencies factor in the entire scope of the research and development program from the analytics to the clinical trial data, which vary on a case-by-case basis.4-6
Extrapolation of Indications
Once the similarity with the reference product has been established in terms of structure, function, pharmacokinetics, pharmacodynamics, efficacy, safety, and immunogenicity, the biosimilar is acknowledged as similar to the reference product. In the event that the reference product is licensed for multiple therapeutic indications, extrapolation of indications may be possible with scientific justification. Extrapolation is defined as the approval of a biosimilar for use in an indication held by the reference product that has not been directly studied in a comparative clinical trial with the biosimilar.11 Regulatory agencies such as the EMA, FDA, and WHO require comprehensive comparability that focuses on efficacy, safety, and immunogenicity with a clinically relevant mechanism of action and receptors in the indication for an extrapolation to be considered.4-6 Additional information may be required if the mechanism of action or receptors involved are different in order to justify the extrapolation of indication.4,6
The utilization of extrapolation is critical to the concept of biosimilarity. The EMA perspective is that “the primary rationale for data extrapolation is to avoid unnecessary studies in the target population for ethical reasons, for efficiency and to allocate resources to areas where studies are the most needed.”12 Weise et al identified replicating the efficacy and safety data of the reference product as scientifically not necessary and even unethical in some cases.13
Within a true biosimilar to the reference product, it is expected that therapeutic effects such as efficacy, safety, and immunogenicity are similar. When extrapolation of clinical data is expected, the therapeutic indication of the clinical studies should be sensitive enough to detect clinically meaningful differences between the proposed biosimilar and the reference product.4-6
Biosimilars approved for cancer therapy have been granted approval for indications that are held by the reference product based on the extrapolation of efficacy and safety data. The following case identifies the scientific data needed to demonstrate a biosimilarity and extrapolation of indication.
Filgrastim (Neupogen): This agent is a granulocyte-colony stimulating factor (G-CSF) used in oncology for supportive care to prevent chemotherapy-induced neutropenia.14 Filgrastim is also utilized in patients with acute myeloid leukemia or severe chronic neutropenia, or who are undergoing bone marrow transplantation and engraftment.14 In the European Union (EU), several biosimilars to filgrastim have been approved, including Biograstim, Filgrastim ratiopharm, Ratiograstim, and Tevagrastim from Teva Ltd (Castleford, UK); Zarzio from Sandoz GmbH (Kundl, Austria); and Nivestim from Hospira (Lake Forest, IL); more recently, Zarxio (filgrastim-sndz) from Sandoz (Princeton, NJ) was approved in the U.S. for all indications of the reference product, Neupogen.15
The justification for the extrapolation of indications for all filgrastim biosimilars consisted of the following: 1) the overall analytical data from a head-to-head comparison of the reference product that showed similar molecular structure and in vitro function, pharmacokinetic studies depicting similar exposure and pharmacodynamics studies depicting an effect on absolute neutrophil and CD34+ cell counts in healthy volunteers, and efficacy, safety, and immunogenicity in cancer patients; and 2) the mechanistic binding to the G-CSF receptor that mediates the same biologic activity (i.e., stimulating bone marrow cells).16 In the case of filgrastim biosimilars, the extrapolation of indications was based on the totality of evidence (i.e., quality, safety, efficacy, and the mechanism of action) of the similarity between filgrastim biosimilars and the reference product, which was further supported by postapproval studies.15,17
Biosimilar mAbs in Development for Cancer Treatment
Considering the high structure complexity of mAbs, the EMA published additional guidelines for the development of mAb biosimilars.18 Essentially, the guideline states that extrapolation of clinical safety and efficacy data to other indications approved for the reference mAb is possible based on the comparability analyses with scientific justification. The request for an indication extrapolation must be scientifically supported in terms of the mechanism of action and the receptors involved in each indication.
In cancer therapy, the typical preferred endpoints to anticancer activity consist of progression-free survival or overall survival, which may not always be sensitive enough to establish similar efficacy of the biosimilar mAbs and the reference products. Hence, the EMA recommends utilizing a clinical endpoint that measures activity as a primary endpoint (i.e., overall response rate or pathologic complete response).18 The EMA also recommends extrapolation of clinical data from a population that is potentially homogeneous and not immune-compromised versus a population that is less homogeneous and is immune-compromised.18
Trastuzumab: Trastuzumab (Herceptin [Genentech and Roche]), a humanized recombinant mAb targeted at the human epidermal growth factor receptor 2 (HER2), is indicated for the treatment of HER2-positive breast cancer in the adjuvant and metastatic setting.19 It is also indicated for the treatment of HER2-positive metastatic gastric or gastroesophageal junction adenocarcinoma. The composition of matter patent covering trastuzumab marketed in Europe (Roche) expired in 2014, while the last composition of matter patent in the U.S. (Genentech) will expire in 2019.20 Trastuzumab biosimilars in late-stage clinical development are focused on metastatic and early breast cancer (TABLE 1).21
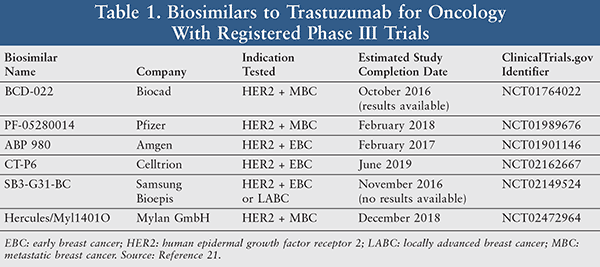
A number of biosimilars to trastuzumab have been approved worldwide. For instance, Herzuma (Celltrion [Incheon City, Republic of Korea]) was approved by the Korean Ministry of Food and Drug Safety in South Korea.22 In addition, Hertraz (Mylan [Mumbai, India]) and CANMAb (Biocon [Bangalore, India]) were approved by the Drug Controller General of India.23 These worldwide biosimilars have been approved for all indications of the reference product Herceptin. Although these biosimilars are approved in Asia, they may not meet the stringent regulatory requirements for clinical justification of biosimilarity guidelines from the EMA, FDA, or WHO.
Bevacizumab: Bevacizumab (Avastin [Genentech and Roche]) is a humanized recombinant mAb targeted at the human vascular endothelial growth factor (VEGF).24,25 In the EU and U.S., bevacizumab is utilized as a constituent of combination therapy in the treatment of metastatic colorectal cancer, metastatic or recurrent nonsquamous non–small-cell lung cancer (NSCLC), and metastatic renal cell carcinoma, in addition to cervical platinum-resistant, recurrent epithelial ovarian, fallopian tube, and primary peritoneal cancers.24 The composition of matter patent covering bevacizumab marketed in the U.S. (Genentech) will expire in 2019, while the last composition of matter patent in Europe (Roche) will expire in 2018.20 There are a few bevacizumab biosimilars in late-stage clinical development (TABLE 2) focused on NSCLC.26 No biosimilars to bevacizumab are currently approved.

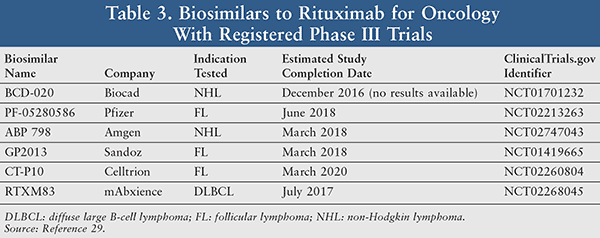
Rituximab: Rituximab (Rituxan [Genentech and Biogen Idec, U.S.]) and MabThera (Roche, EU) are chimeric murine human mAbs targeted at the CD20 antigen of B cells. Rituximab contains a dual therapeutic area of oncology and anti-inflammation. Rituximab is utilized as a constituent in combination with glucocorticoids for treatment of non-Hodgkin lymphoma, chronic lymphocytic leukemia, rheumatoid arthritis, and granulomatosis with polyangiitis and microscopic polyangiitis.27,28 The composition of matter patent covering rituximab marketed in Europe (MabThera) expired in 2013, while the last composition of matter patent in the U.S. (Rituxan) will expire in 2018.20
A number of rituximab biosimilars are in late-stage clinical development (TABLE 3) and are focused on various indications such as rheumatoid arthritis, follicular lymphoma, and diffuse large B-cell lymphoma.29 A biosimilar to rituximab has been approved by the Russian Ministry of Health (AcellBia [Biocad, St. Petersburg, Russia]).30
Conclusion
As biosimilar mAbs begin to enter the field of oncology, it is increasingly important for cancer practitioners to understand the biosimilar development and evaluation process of data in order to make an informed decision and incorporate these medications into clinical practice. With a true biosimilar to the reference product, it is expected that therapeutic effects such as efficacy, safety, and immunogenicity are similar. The approval of biosimilar mAbs for oncologic therapeutics is expected because patents for oncology biologic mAbs have already expired or will expire in coming years.
Biosimilarity is based on comparable analytics and functional, nonclinical, and clinical studies. The extrapolation of an indication is an important component of the biosimilar concept. The mAb biosimilar development program’s utilization of extrapolation may address drug shortage issues (i.e., increased demand and manufacturing) within the stringent regulatory requirements of the EMA, FDA, and WHO. The European experiences with biosimilars have shown promise over the past few years with similarities to the reference biologic. Oncology pharmacists can play a pivotal role in the continued and increased use of biosimilars in cancer therapy.
REFERENCES
1. Baer IW, Maini A, Jacobs I. Barriers to the access and use of rituximab in patients with non-Hodgkin’s lymphoma and chronic lymphocytic leukemia: a physician survey. Pharmaceuticals (Basel). 2014;7(5):530-544.
2. Lammers P, Criscitiello C, Curigliano G, et al. Barriers to the use of trastuzumab for HER2+ breast cancer and the potential impact of biosimilars: a physician survey in the United States and emerging markets. Pharmaceuticals (Basel). 2014;7(9):943-953.
3. Cornes P. The economic pressures for biosimilar drug use in cancer medicine. Target Oncol. 2012;7(suppl 1):S57-S67.
4. European Medicines Agency. Guideline on similar biological medicinal products. October 2014. www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2014/10/WC500176768.pdf. Accessed November 1, 2016.
5. FDA. Scientific considerations in demonstrating biosimilarity to a reference product. Guidance for industry. April 2015. www.fda.gov/downloads/drugs/guidancecomplianceregulatoryinformation/guidances/ucm291128.pdf. Accessed November 1, 2016.
6. World Health Organization (WHO). Expert Committee on Biological Standardization. Guidelines on evaluation of similar biotherapeutic products (SBPs). October 2009. www.who.int/biologicals/areas/biological_therapeutics/BIOTHERAPEUTICS_FOR_WEB_22APRIL2010.pdf. Accessed November 1, 2016.
7. Dörner T, Strand V, Cornes P, et al. The changing landscape of biosimilars in rheumatology. Ann Rheum Dis. 2016;75(6):974-982.
8. Rugo HS, Linton KM, Cervi P, et al. A clinician’s guide to biosimilars in oncology. Cancer Treat Rev. 2016;46(1):73-79.
9. European Medicines Agency. European Public Assessment Report (EPAR) summary for the public: Filgrastim Hexal. Updated July 2014. www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Summary_for_the_public/human/000918/WC500022469.pdf. Accessed January 9, 2017.
10. Center for Drug Evaluation and Research. Summary review. Zarxio/Filgrastim-sndz. March 6, 2015. www.accessdata.fda.gov/drugsatfda_docs/nda/2015/125553Orig1s000SumR.pdf. Accessed January 10, 2017.
11. Curigliano G, O’Connor DP, Rosenberg JA, Jacobs I, et al. Biosimilars: extrapolation for oncology. Crit Rev Oncol Hematol. 2016;104(3):131-137.
12. European Medicines Agency. Concept paper on extrapolation of efficacy and safety in medicine development: final. March 19, 2013. www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2013/04/WC500142358.pdf. Accessed November 1, 2016.
13. Weise M, Bielsky MC, De Smet K, et al. Biosimilars: what clinicians should know. Blood. 2012;120(26):5111-5117.
14. Neupogen (filgrastim) prescribing information. Thousand Oaks, CA: Amgen Inc; June 2016. http://pi.amgen.com/~/media/amgen/repositorysites/pi-amgen-com/neupogen/neupogen_pi_hcp_english.ashx. Accessed January 9, 2017.
15. Gascón P, Tesch H, Verpoort K, et al. Clinical experience with Zarzio in Europe: what have we learned? Support Care Cancer. 2013;21(10):2925-2932.
16. Gascón P. Presently available biosimilars in hematology-oncology: G-CSF. Target Oncol. 2012;7(1):29-34.
17. Schmitt M, Publicover A, Orchard KH, et al. Biosimilar G-CSF based mobilization of peripheral blood hematopoietic stem cells for autologous and allogeneic stem cell transplantation. Theranostics. 2014;4(3):280-289.
18. European Medicines Agency. Guideline on similar biological medicinal products containing monoclonal antibodies—non-clinical and clinical issues. May 30, 2012. www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2012/06/WC500128686.pdf. Accessed November 1, 2016.
19. Herceptin (trastuzumab) prescribing information. South San Francisco, CA: Genentech Inc; March 2016. www.gene.com/download/pdf/herceptin_prescribing.pdf. Accessed November 1, 2016.
20. Philippidis A. Biosimilars: 11 drugs to watch. Genetic Engineering & Biotechnology News (GEN). May 19, 2014. www.genengnews.com/the-lists/biosimilars-11-drugs-to-watch/77900135. Accessed January 9, 2017.
21. Trastuzumab biosimilar. www.clinicaltrials.gov. Accessed January 9, 2017.
22. Generics and Biosimilars Initiative (GaBI) Online. Biosimilar trastuzumab approved in Korea. January 17, 2014. www.gabionline.net/Biosimilars/News/Biosimilar-trastuzumab-approved-in-Korea. Accessed November 1, 2016.
23. GaBI Online. Mylan launches trastuzumab ‘similar biologic’ in India. February 7, 2014. www.gabionline.net/Biosimilars/News/Mylan-launches-trastuzumab-similar-biologic-in-India. Accessed November 1, 2016.
24. Avastin (bevacizumab) prescribing information. South San Francisco, CA: Genentech Inc; December 2016. www.gene.com/download/pdf/avastin_prescribing.pdf. Accessed January 9, 2017.
25. European Medicines Agency. EPAR summary for the public: Avastin. Updated May 2016. www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Summary_for_the_public/human/000582/WC500029260.pdf. Accessed January 9, 2017.
26. Bevacizumab biosimilar. www.clinicaltrials.gov. Accessed January 9, 2017.
27. Rituxan (rituximab) prescribing information. South San Francisco, CA: Genentech, Inc; April 2016. www.gene.com/download/pdf/rituxan_prescribing.pdf. Accessed January 9, 2017.
28. European Medicines Agency. EPAR summary for the public: MabThera. Updated June 2016. www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Summary_for_the_public/human/000165/WC500025815.pdf. Accessed January 9, 2017.
29. Rituximab biosimilar. www.clinicaltrials.gov. Accessed January 9, 2017.
30. Kumar R, Sigala S, Malgarini RB, et al. Biosimilars: regulatory status and implications across the world. J Pharmacovigil. 2015;S3:002.
To comment on this article, contact rdavidson@uspharmacist.com.






