ABSTRACT: Gone are the anxiety-filled days of stay-at-home orders, strict social distancing, and mask mandates to protect ourselves from the transmission of COVID-19. Yet, the risk for contracting COVID-19 is persistent. After years of COVID-19, there are evidence-based guidelines to enable clinicians to best treat their patients. These guidelines are living, and, therefore, they are reviewed and updated regularly with the best available evidence and expert opinion. Guidelines agree that patients with mild disease who have a high risk for disease progression can be treated within the first few days of symptom onset with various therapies.
As healthcare providers, it is imperative to be knowledgeable about drug therapies for ambulatory COVID-19 adult patients. With pharmacists as an easy healthcare access point, patients may seek out additional information about COVID-19 treatment options. In some situations, treatment may be dispensed via a community pharmacy. Detailed guidelines are published by the World Health Organization (WHO), the National Institutes of Health (NIH), and the Infectious Diseases Society of America (IDSA). The mentioned guidelines are living and are therefore reviewed and updated regularly with the best available evidence and expert opinion. The guidelines differ based on disease-severity staging, evidence rating system, and overall recommendations. All guidelines agree that the medications to consider for ambulatory adult patients at high risk for COVID-19 progression are nirmatrelvir/ritonavir, remdesivir, and molnupiravir.
The three guidelines each have a different classification system to stratify ambulatory patients with COVID-19. The WHO defines nonsevere COVID-19 as the absence of acute respiratory distress syndrome, sepsis, septic shock, oxygen saturation <90% on room air, signs of pneumonia, or signs of respiratory distress. They define patients as highest risk for hospitalization as those with older age, immunosuppression, and/or chronic diseases, with lack of COVID-19 vaccination as an additional risk factor.1 Chronic diseases include hypertension, diabetes, cardiac disease, chronic lung disease, cerebrovascular disease, dementia, mental disorders, chronic kidney disease, obesity, and cancer.2 In comparison with the WHO, the NIH has two different taxonomies for ambulatory patients with COVID-19. The NIH defines ambulatory patients as mild or moderate. Patients presenting with mild illness have signs and symptoms of COVID-19 but do not have shortness of breath, dyspnea, or abnormal chest imaging. Individuals who fall in the moderate illness category show evidence of lower respiratory disease and have a pulse oximetry ≥94% on room air at sea level.3 Similarly to the NIH, the IDSA divides patients into mild and moderate disease categories. The IDSA defines mild disease as symptoms suggestive of upper respiratory tract involvement without features of lung or other end-organ involvement. Moderate disease occurs when there is pulmonary involvement but no hypoxia.4 See TABLE 1 for the respective treatment guidelines.1,2,4
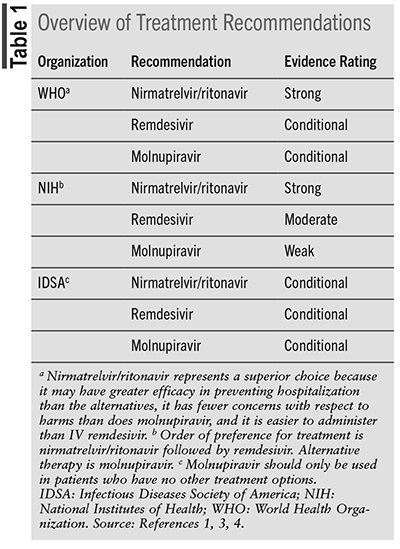
The WHO, NIH, and IDSA guidelines are noted to be living guidelines. Living guidelines are constantly under review, acquiring new evidence and publishing recommendations as quickly as possible. The three organizations use a similar approach to collecting evidence, reviewing it, and making recommendations. The guidelines differ, however, in how they classify their recommendations.
The WHO uses the GRADE approach to quantify evidence certainty and strength of recommendations. The recommendations are then rated. A strong recommendation is defined as when benefits outweigh the harms for almost everyone. It is thought that all or nearly all informed patients would likely want this option.1 Below strong in the hierarchy of recommendation strength is a conditional recommendation. A conditional recommendation is defined as benefits outweighing harms for the majority but not all patients.1
The NIH guideline recommendations are based on scientific evidence and expert opinion. Each recommendation includes a capital letter and a Roman numeral. The capital letter identifies the strength of the recommendation. The Roman numeral identifies the quality of evidence that supports the recommendation.3 Strength of recommendations are classified as A, B, or C, equating to strong, moderate, and weak, respectively. Roman numeral I indicates a high quality of evidence. High-quality evidence is one or more randomized trials without major limitations, well-powered subgroup analyses, or meta-analyses without major limitations. Roman numeral II equates to a moderate quality of evidence. This is further broken down into IIa and IIb recommendations. IIa recommendations are moderate-quality evidence from randomized trials and subgroup analyses that do not meet the criteria for a I rating. IIb recommendations are moderate-quality evidence of observational studies without major limitations. Roman numeral III indicates expert opinion.3
When the IDSA reviews evidence to make recommendations, the panel uses the GRADE evidence in the decision process, including the certainty of evidence and the balance between desirable and undesirable effects.4 Published recommendations use the words “recommend” and “suggest,” relaying the strength of the recommendation to the reader. The word “recommend” is a strong recommendation, implying that most people would want the course of action and only a small population would not. The word “suggest” indicates a conditional recommendation that implies the majority of people would want the recommended course of action, but many would not.4
Nirmatrelvir/Ritonavir
The WHO, NIH, and IDSA recommend nirmatrelvir/ritonavir, remdesivir, and molnupiravir for treating ambulatory patients with COVID-19. Nirmatrelvir/ritonavir is the preferred therapy. The WHO makes a strong recommendation for treatment with this agent to patients with nonsevere COVID-19 at highest risk for hospitalization.1 The evidence to support this decision was based on two trials. The data showed that nirmatrelvir/ritonavir likely reduces hospital admission with little or no impact on mortality, and treatment does not increase the likelihood of adverse effects leading to drug discontinuation.1 Certainty of the evidence related to hospitalization is rated as moderate due to concerns regarding imprecision and risk of bias. The evidence for mortality was rated as low due to serious imprecision and indirectness of the trials. The evidence surrounding minimal drug discontinuation due to adverse effects was rated as high.1 The NIH states that nirmatrelvir/ritonavir is the preferred therapy for patients who have mild-to-moderate COVID-19 who do not require supplemental oxygen and are at high risk of progression to severe disease.3 Evidence for nirmatrelvir/ritonavir is rated as AIIa, equating to a strong recommendation with moderate quality of evidence based on randomized trials. Nirmatrelvir/ritonavir is first line because it has high efficacy, has been shown to reduce hospitalization and death, and is an oral medication.3 The IDSA comments on nirmatrelvir/ritonavir in recommendation 26. It states, “In ambulatory patients with mild-to-moderate COVID-19 at high risk for progression to severe disease, the IDSA guidelines suggests nirmatrelvir/ritonavir initiated within 5 days of symptom onset rather than no nirmatrelvir/ritonavir.” This is classified as a conditional recommendation with low certainty evidence.4 Overall, both the WHO and the NIH provide a strong recommendation in favor of nirmatrelvir/ritonavir. The IDSA is slightly more conservative and provides a conditional recommendation.
Nirmatrelvir/ritonavir is branded as Paxlovid and manufactured by Pfizer. Nirmatrelvir is an inhibitor to the main protease of SARS-CoV-2. Inhibition of this protease blocks viral replication.4 Nirmatrelvir has demonstrated antiviral activity against all coronaviruses that are known to infect humans.3 Ritonavir is a strong CYP450 3A4 inhibitor that is used as a pharmacokinetic-boosting agent. The addition of ritonavir increases the concentrations of nirmatrelvir to target.3 Ritonavir does not exert any direct antiviral activity itself.1
Nirmatrelvir/ritonavir should be initiated as soon as possible after COVID-19 diagnosis and within 5 days of symptom onset.5 The dosage to start the patient on is based on estimated glomerular filtration rate (eGFR). Patients with an eGFR ≥60mL/min should be started on standard dosing, which is nirmatrelvir 300 mg and ritonavir 100 mg twice daily for 5 days.5 Patients with moderate renal impairment defined as eGFR ≥30 to <60 mL/min should use a reduced dosage of nirmatrelvir 150 mg and ritonavir 100 mg twice a day for 5 days.5 Patients with an eGFR <30 mL/min are contraindicated to use nirmatrelvir/ritonavir.5 The nirmatrelvir/ritonavir course of therapy is supplied as a dose pack. The pack contains two types of tablets—pink nirmatrelvir tablets and white ritonavir tablets.6 The blister dose pack is clearly marked with the time of day to take each tablet. Based on the dosage prescribed, patients will be taking two or three tablets total per dose.
Nirmatrelvir/ritonavir is not indicated for certain patient populations. It is not recommended for people with severe hepatic impairment (Child-Pugh class C). It is contraindicated with coadministration with drugs highly dependent on CYP3A for clearance and for which elevated concentrations are associated with serious or life-threatening reactions (e.g., alfuzosin, ranolazine, amiodarone, simvastatin). It is also contraindicated with potent CYP3A inducers (e.g., St. John’s wort, rifampin, phenytoin) where significantly reduced nirmatrelvir or ritonavir plasma concentrations may be associated with the potential for loss of virologic response and possible resistance.3,5
One of the main concerns with nirmatrelvir/ritonavir is the significant number of drug-drug interactions due to ritonavir. The drug interaction will last the entire 5-day treatment duration and at least 2 to 3 days after completion. The time could be extended in adults of advanced age or with drugs that have a longer half-life.3 The NIH guideline details medications that have clinically relevant interactions. It also recommends a Web-based drug-drug interaction checker created by the Department of Pharmacology at the University of Liverpool.3,7 The guideline further supplies additional interactive links to other published tables and guidance for managing drug-drug interactions.3 Prescribers and dispensers should review these resources prior to providing patients with nirmatrelvir/ritonavir to ensure patient safety.
Patients and providers should be aware that nirmatrelvir/ritonavir has the potential to cause hepatic transaminase elevations, clinical hepatitis, and jaundice.5 Hypersensitivity reactions have also been reported to include angioedema, anaphylaxis, toxic epidermal necrolysis, and Stevens-Johnson syndrome.5 Side effects include alterations of taste, diarrhea, hypertension, and myalgia.3
Remdesivir
The WHO endorses remdesivir with a conditional recommendation for treatment of patients with nonsevere COVID-19 at highest risk for hospitalization.1 When assessing the evidence from five trials, the WHO noted that remdesivir probably reduces hospital admission and may have little or no impact on mortality. The treatment probably does not increase the likelihood of adverse effects leading to drug discontinuation.1 The certainty of the evidence related to reduced hospital admission is rated as moderate due to serious imprecision. When considering the mortality conclusions, the evidence rating is low because of serious imprecision and indirectness. Unlike the other drugs considered for nonsevere COVID-19, the evidence around treatment discontinuation due to adverse effects is rated as moderate.1 The NIH places remdesivir as second-line therapy after nirmatrelvir/ritonavir for nonhospitalized adults with mild-to-moderate COVID-19. Remdesivir is rated as BIIa, meaning a moderate recommendation with moderate-quality evidence based on randomized trials. The recommendation is based on a phase III randomized, placebo-controlled trial that reported high clinical efficacy in high-risk patients who were unvaccinated.3 IDSA guidelines mention remdesivir in recommendation 15, stating that among ambulatory or hospitalized patients with mild-to-moderate COVID-19 at high risk for progression to severe disease, “the IDSA guideline panel suggests remdesivir initiated within 7 days of symptom onset rather than no remdesivir.” This is a conditional recommendation with low certainty of evidence.4
Remdesivir is branded as Veklury, manufactured by Gilead. Remdesivir is an antiviral drug that is a nucleotide prodrug of an adenosine analog. It inhibits viral replication by terminating RNA transcription prematurely.3 Remdesivir was initially developed for hepatitis C infection. It has been studied for multiple other viruses, such as Ebola and Marburg viral infections.1
In nonhospitalized patients with high risk of progression to severe illness, remdesivir is dosed as 200 mg IV on Day 1 followed by 100 mg IV once daily on Days 2 and 3. The medication should be started as soon as possible after COVID-19 diagnosis and within 7 days of symptom onset.3 Patients should have an eGFR ≥30 mL/min to receive therapy.
Remdesivir is available as a 100-mg/20-mL solution in vial or as a lyophilized powder that must be reconstituted with 19 mL of sterile water for injection.9 The resulting solution is further diluted into 100 mL or 250 mL of normal saline prior to administration. The final product is stable for 24 hours at room temperature or 48 hours under refrigeration. The IV infusion should be administered under conditions where severe hypersensitivity reactions can be managed. Infusion time can be set anywhere between 30 and 120 minutes.8 Hypersensitivity reactions have been observed. Slower infusion rates can be considered to reduce the risk of the reaction. Patients should be monitored during infusions and observed for at least 1 hour after infusion completion.9
Additionally, remdesivir carries a risk for an increase in transaminases. In healthy patients, reversible transaminase elevations have been observed. It is recommended to perform hepatic laboratory testing in all patients before starting and while receiving treatment. Providers should consider discontinuation of remdesivir if ALT levels increase to greater than 10 times the upper limit of normal. However, remdesivir should be discontinued if an ALT elevation is accompanied by signs or symptoms of liver inflammation.9 The manufacturer also recommends providers review prothrombin time prior to initiating therapy, as a side effect of the medication is prolonged prothrombin time.9 Other side effects include nausea, increased serum glucose, decreased creatinine clearance, and potentially severe bradycardia (some fatal).10,11
Molnupiravir
The WHO guidelines conditionally recommend molnupiravir for treating patients with nonsevere COVID-19 at highest risk for hospitalization. Children and women who are pregnant or breastfeeding are excluded from this recommendation.1 The evidence states the molnupiravir probably reduces hospital admission and time to symptom resolution, and it may reduce mortality. Treatment does not increase the likelihood of adverse effects leading to drug discontinuation.1
The major concern surrounding molnupiravir is the potential for long-term harm based on the risk of mutagenesis. The recommendations for molnupiravir were based on six studies. Certainty of evidence surrounding reduced hospital admission and time to symptom resolution is rated as moderate because of serious imprecision surrounding hospital admission and a risk of bias for symptom resolution. The evidence to support reduced mortality is rated as low certainty because of serious imprecision and indirectness. The evidence surrounding minimal drug discontinuation due to adverse effects was rated as high.1 In the NIH guidelines, molnupiravir is rated as CIIa, a weak recommendation based on moderate quality of evidence from randomized trials. The medication is not preferred because molnupiravir appears to have lower clinical efficacy than the other treatments, although head-to-head trials are lacking.3 IDSA recommendation 27 states, “In ambulatory patients (≥18 years) with mild-to-moderate COVID-19 at high risk for progression to severe disease who have no other treatment options, the IDSA guideline panel suggests molnupiravir initiated within five days of symptom onset rather than no molnupiravir.” This is considered a conditional recommendation with low certainty of evidence.4
Molnupiravir is branded as Lagevrio by Merck. Molnupiravir is an oral prodrug that after conversion will be incorporated into viral RNA as a nucleoside analogue.1 Serial mutations will develop resulting in viral inhibition by mutagenesis.12
For treating mild-to-moderate COVID-19 with high risk of progression to severe illness, molnupiravir is dosed at 800 mg every 12 hours for 5 days. Molnupiravir is available as 200-mg capsules, or one dose equating to four capsules. Molnupiravir must be initiated as soon as possible after COVID-19 diagnosis and within 5 days of symptom onset.1 The drug is well tolerated overall with common side effects of diarrhea, nausea, and dizziness.12
Molnupiravir is only indicated for patients aged ≥18 years because of the risk for bone and cartilage toxicity. This effect on bone and cartilage growth was observed in rats after repeat dosing.12 Molnupiravir is not recommended to use during pregnancy due to risks for embryo-fetal toxicity.12 Assessment of pregnancy status should be performed for women of childbearing age with consideration to pregnancy testing when clinically indicated.12 Contraception is recommended for patients during and after treatment if there is a potential for conception. Females should use contraception for the duration of molnupiravir therapy and for 4 days after the last dose of medication. They should also refrain from breastfeeding during this time if lactating. Males should use a reliable method of contraception correctly and consistently during treatment and for at least 3 months after the last dose. The risk is regarded as low; however, nonclinical studies of the drug’s effect on offspring of treated males have not been completed.12
Conclusion
COVID-19 guidelines are published by multiple different organizations and are continuously evolving. The three drugs that are recommended for ambulatory COVID-19 patients with a high risk for disease progression include nirmatrelvir/ritonavir, remdesivir, and molnupiravir. The guidelines endorse these medications at different strengths of recommendation based on their evaluation of the available evidence. Each treatment regimen is unique. Nirmatrelvir/ritonavir is available as an oral dose pack for ease of administration, but it requires patients to take multiple tablets per dose, and it has numerous significant drug interactions. Remdesivir has the shortest treatment duration of only 3 days; however, the medication is only available in an intravenous formulation. Molnupiravir is overall well tolerated but has a concerning side effect for bone and cartilage toxicity. To minimize the risk, there are detailed recommendations for contraception in both men and women.
Although a positive COVID-19 test still strikes fear and anxiety in patients and healthcare workers alike, they can rest assured that there are clinical guidelines and prescription treatment options available when indicated.
REFERENCES
1. World Health Organization. Therapeutics and COVID-19: living guideline. Geneva: World Health Organization. www.who.int/publications/i/item/WHO-2019-nCoV-therapeutics-2022.4. Accessed January 13, 2023.
2. World Health Organization. Remdesivir for COVID-19. 2022. https://apps.who.int/iris/handle/10665/359753. Accessed March 8, 2023.
3. NIH. Coronavirus disease 2019 (COVID-19) treatment guidelines. www.covid19treatmentguidelines.nih.gov/. Accessed January 9, 2023.
4. Bhimraj A, Morgan RL, Shumaker AH, et al. Infectious Diseases Society of America guidelines on the treatment and management of patients with COVID-19. Infectious Diseases Society of America 2023; version 10.2.1. www.idsociety.org/practice-guideline/covid-19-guideline-treatment-and-management/. Accessed March 27, 2023.
5. FDA. Fact sheet for healthcare providers: emergency use authorization for Paxlovid (nirmatrelvir and ritonavir). Revised September 26, 2022. www.fda.gov/media/155050/download. Accessed January 23, 2023.
6. Paxlovid. How to take Paxlovid (nirmatrelvir tablets; ritonavir tablets). www.paxlovid.com/how-to-take. Accessed January 23, 2023.
7. Liverpool Drug Interactions Group. COVID-19 drug interactions. https://covid19-druginteractions.org/about. Accessed January 23, 2023.
8. Veklury (remdesivir) dosing and administration. www.vekluryhcp.com/dosing-and-admin/. Accessed January 23, 2023.
9. Veklury (remdesivir) product information. www.gilead.com//media/files/pdfs/medicines/covid-19/veklury/veklury_pi.pdf. Revised December 2022. Accessed January 23, 2023.
10. Remdesivir. In: Lexi-Drugs. Lexicomp; 2023. Updated January 13, 2023. http://online.lexi.com. Accessed January 23, 2023.
11. Touafchia A, Bagheri H, Carrié D, et al. Serious bradycardia and remdesivir for coronavirus 2019 (COVID-19): a new safety concerns. Clin Microbiol Infect. 2021;27(5):791.e5-791.e8.
12. FDA. Fact sheet for healthcare providers: emergency use authorization for Lagevrio (molnupiravir) capsules. www.fda.gov/media/155054/download. Accessed March 9, 2023.
The content contained in this article is for informational purposes only. The content is not intended to be a substitute for professional advice. Reliance on any information provided in this article is solely at your own risk.
To comment on this article, contact rdavidson@uspharmacist.com.






