US Pharm. 2024;49(11):6-9.
ABSTRACT: Pharmacists have established positive and powerful roles that are integral to the medication management of diabetes. With the evolution of new tools in their clinical toolbox, they are now also able to expand their clinical success into the optimization of technology using continuous glucose monitoring (CGM) to improve diabetic outcomes even more. With their accessibility in both the community and hospital settings, pharmacists can help bridge the fear and uncertainty of initial CGM use as well as provide patients with ongoing support to assist in obtaining reliable and accurate monitoring results to help navigate their overall diabetes care plan.
Before the significance of the technological advancements that continuous glucose monitoring (CGM) offers can be appreciated, it is helpful to reflect on past milestones that drove therapeutic treatment decisions. Efforts to use the physiologic measurement of glucose date to the time of the ancient Egyptians; however, the value of clinical detection became notable in 1776 when the British physiologist Matthew Dobson reported his experiential findings that diabetic urine was sweet as a result of its sugars.1,2 The true launch of glucose testing, however, is recognized to be the use of copper reagents, first reported in 1908, to detect urine glucose. This set the stage for developments that would spark the release of the first glucose meter.3,4
Although urine tests were inexpensive and offered fast results, they were associated with a great degree of imprecision, with false-positives and false-negatives frequently observed. These tests were also prone to highly subjective and variable human interpretation.1 The first reports of measuring glucose in the blood were in 1957, with a point-of-care (POC) device developed by Joachim Kohn, who discovered that approximate levels of blood glucose could be measured using Clinistix, a urine reagent strip that contained orthotolidine, peroxidase, and glucose oxidase.1,5 Later developments included strips created specifically for use with blood that translated results into color changes and charts to measure blood glucose.1
The development of the first prototype by the Ames Corporation in 1966 was a milestone in POC glucose monitoring, as it helped expand the growth of these devices in the 1980s. The Ames Reflectance Meter continued to use what is known as Dextrostix technology, which measured glucose with reflectance photometry and displayed the approximate glucose reading as either 0–4 mmol/L, 4–10 mmol/L, or 10–55 mmol/L.1,6 Unfortunately, this prototype was also fraught with inaccuracy and was expensive and cumbersome.1
Building on Current Blood Glucose Measurement
More current versions of blood glucose monitoring (BGM) continued to subject patients to daily, or more frequent, needle sticks; however, these versions did help improve earlier recognition of hypoglycemic episodes, confirm excursions of hyperglycemia, or plan prandial insulin doses in real time. Equipment failure and human error, such as improper strip handling or device use, however, have continued to create the need for improved technology and monitoring options.7,8
Critics of personal BGM may prefer the use of glycosylated hemoglobin A1C, which estimates the average blood glucose over the preceding 8 to 12 weeks and is considered a standard-of-care monitoring tool. The use of A1C, however, may also have false-positive high values in certain patients and under specific conditions, but more importantly, A1C serves no role in detecting real-time hypoglycemic events, which can result in serious clinical consequences.8-10
Moodley and colleagues, in their 2015 publication, forecast that future POC devices would need to incorporate data management and connectedness to advanced information technology as well as offer reliable noninvasive methods and patient comfort to improve adherence.1 Almost 10 years later, CGM offer various technologic benefits to the healthcare community today.
CGM Devices Offer Flexibility
CGM devices are available for both personal and professional use, with the latter being the property of and used by the prescriber at the patient’s clinic appointment. The patient is instructed to wear the device for a specific period, such as 1 or 2 weeks at a time. The prescriber may choose to have data be visible or not visible to the patient wearing the device. Conversely, the patient is the owner of his or her personal CGM device, which is intended for a more frequent, even continuous, use as a real-time CGM (rtCGM) that continuously stores glucose levels that it measures without prompting.11
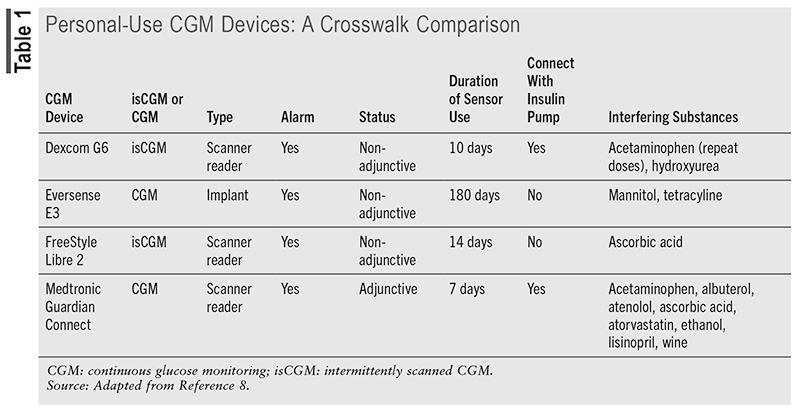
There are also intermittent-scanning CGM devices (isCGM) that require scanning to store the values. Adjunctive personal-use CGM devices can make insulin dose changes, but only after BGM confirms the values, whereas nonadjunctive devices do not require BGM value confirmation to make changes.12,13 Of the devices listed in TABLE 1, which are all personal-use CGM devices, only the Medtronic Guardian Connect is an adjunctive device that requires further confirmation to make insulin dose changes. Other differences are noted; however, they all offer real-time remote monitoring and are equipped with alarm alerts.8
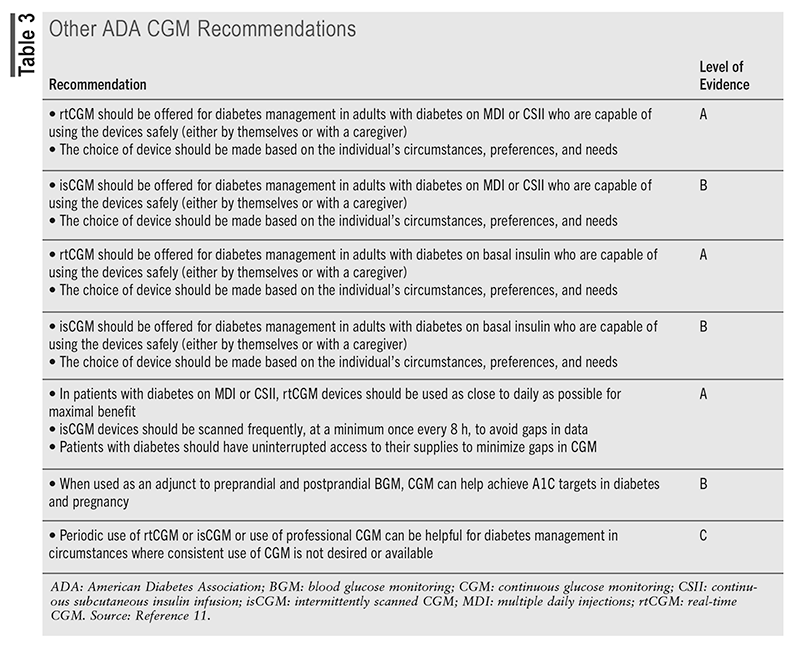
The American Diabetes Association (ADA) recommends that devices be offered to patients with diabetes, and the initiation of CGM should be offered to patients with type 1 diabetes (T1D) early in the disease, even at the time of diagnosis.11 The ADA recommendations are assigned ratings of A, B, or C, depending on the quality of evidence, with E as a separate category for recommendations for which there is no evidence from clinical trials but are instead based on expert opinion. Recommendations with A ratings are weighted the heaviest and are considered to have the greatest likelihood of improving clinical outcomes since they are generally based on large, well-designed clinical trials or meta-analyses.13
Establishing CGM Competency
To ensure success, the ADA suggests establishing competencies for healthcare professionals who work with diabetes technology. The type and selection of devices should also be individualized based on a patient’s specific needs, preferences, and skill level. In the setting of an individual whose diabetes is co-managed or managed solely by someone else (e.g., a young child or a person with cognitive impairment or dexterity, psychosocial, and/or physical limitations), the caregiver’s skills and preferences are also essential and should be factored into the decision-making process.11 As is the case with any new technology, the level of interest and degree of intimidation felt by the potential user will impact the ultimate success of the intervention. Additionally, variable insurance coverage and prescriber familiarity or uncertainty also play a role in the selection of the CGM device.
The ADA recommends https://consumerguide.diabetes.org as a resource to assist both the patient and healthcare professional in making initial decisions about devices if they are unsure about the available options.11 They further advise that when prescribing or dispensing a device, patients with diabetes and their caregivers should receive initial and ongoing education and training, either in person or remotely. Ongoing evaluation of technique, results, and the ability to utilize and interpret data—including uploading/sharing data (if applicable) to monitor and adjust therapy—should also take place.11
The diagnosis and disease management involved in diabetes care, as with any chronic disease, can be overwhelming, and ongoing support is key to therapeutic success. Initial training competency may not be retained until the device has been used routinely by the patient. Even with routine use, challenges may arise and dysfunction may occur that require troubleshooting by healthcare professional coaches or device manufacturers. The ADA advises that ongoing education and training be offered in person or remotely.
Another recommendation by the ADA is to ensure that patients are informed to self-monitor for any dermatologic adverse effects, which can be due to either irritation or allergic reaction, when using CGM devices. Contact dermatitis associated with the ingredients in the tape adhesive and other chemical exposures involved in CGM use may develop over time.11,14 Additionally, pharmacists can warn patients about the potential of seemingly benign substances that may interfere with the accuracy of results, such as acetaminophen and ascorbic acid, among others (see TABLE 1).8,15,16 Providers should review manufacturer data and emerging literature for potentially interacting medications.
Pediatric CGM Recommendations
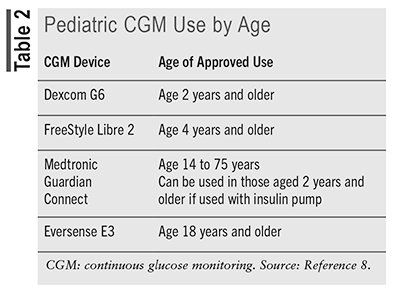
Departing slightly from the broad adult recommendations that rtCGM or isCGM be used for the management of diabetes, regardless of the type, the ADA puts forth a higher level of evidence for use in pediatric patients with T1D.11 The ADA advises that rtCGM (evidence grade A) or isCGM (evidence level E) should be offered to manage diabetes in youth who are diagnosed with T1D, who receive multiple daily insulin injections or continuous subcutaneous insulin infusion (via pump), and who are capable of using the device either on their own or with the support of a caregiver. Offering either rtCGM or isCGM to manage type 2 diabetes (T2D) in youth was recommended at an evidence grade E; however, whether for managing T1D or T2D, the ADA strongly urges that the device choice be made based on consideration of the patient’s preferences, needs, and circumstances. Although the ADA 2024 standards do not specify an age range for their definition of “youth,” the available devices clearly indicate the age in years for appropriate use of the CGM devices (see TABLE 2).8
The ADA strongly advises that individuals with diabetes who have been using CGM or other diabetes technologies have continued access across third-party payers, regardless of age or A1C levels (see TABLE 3).11 This, however, is not always the case. Pharmacists can recognize insurance barriers and assist the patient and prescriber in overcoming these hurdles.
The FDA approved an OTC CGM system that is expected to remove the barrier and burden of obtaining a prescription to benefit from this clinical tool. In May 2024, the American Journal of Managed Care (AJMC) indicated that advancements over the past 2 decades have made CGM more user friendly with smaller sensors, decreased challenges of calibration with fingerstick monitoring, and overall device operability. Despite this advocacy, as reported in the AJMC, CGM remains underutilized in primary care, and most patients with T2D are treated in primary care settings.17
In March 2023, the Center for Medicare & Medicaid Services announced expanded coverage of CGM for Medicare beneficiaries using any insulin regimen or for patients who are not on insulin but with a history of problematic hypoglycemia.18 This coverage approval was based on the growing body of evidence supporting the effectiveness of CGM in patients with T2D. Landmark support for CGM also occurred in 2023 with the ADA, the American Academy of Clinical Endocrinologists, and the Endocrine Society updating their respective clinical practice guidelines to recommend the use of CGM.19,20 The changes to these guidelines included a recommended increased use of CGM in patients with T2D and broadened access to CGM technology.
Conclusion
The CDC estimated that in 2019, 37.3 million people (11.3% of the U.S. population) were diagnosed with diabetes, with likely 25% more (8.5 million) undiagnosed. Prediction models inform that by 2050, one in three Americans will be diagnosed with diabetes during their lifetime. With this staggering statistic, it becomes even more critical to optimize the use of diabetes technology, including devices such as CGM and the hardware and software needed to empower individuals diagnosed with diabetes to self-manage their condition.
Diabetes technology has broadened to include “CGM-informed algorithms” that modulate automatic insulin administration. For these technological advances to be successful, their use must also involve education and ongoing support, as their sophistication and their evolution continue to expand. Pharmacists can strengthen this resolve to embrace technology for optimal use by promoting access to appropriate patients, collaborating to identify and resolve health inequities and social determinants that negatively affect individuals diagnosed with diabetes. Pharmacists should stay current with guideline recommendations and emergency data that inform the healthcare community of the clinical benefits of optimal use of CGM and other diabetes technologies as they become available.
REFERENCES
1. Moodley N, Ngxamngxa U, Turzyniecka MJ, Pillay TS. Historical perspectives in clinical pathology: a history of glucose measurement. J Clin Pathol. 2015;68(4):258-264.
2. Matthew Dobson (1735-1784) clinical investigator of diabetes mellitus. JAMA. 1968;205:698.
3. Hirsch IB. Introduction: history of glucose monitoring. In: Role of Continuous Glucose Monitoring in Diabetes Treatment. Arlington, VA: American Diabetes Association; 2018.4. Clarke SF, Foster JR. A history of blood glucose meters and their role in self-monitoring of diabetes mellitus. Br J Biomed Sci. 2012;69:83-93.
5. Kohn J. A rapid method of estimating blood-glucose ranges. Lancet. 1957;273:119-121.
6. Cheah JS, Wong AF. A rapid and simple blood sugar determination using the Ames reflectance meter and Dextrostix system: a preliminary report. Singapore Med J. 1974;15:51-52.7. Klonoff DC. Benefits and limitations of self-monitoring of blood glucose. J Diabetes Sci Technol. 2007;1(1):130-132.
8. Unger J. Continuous glucose monitoring overview: features and evidence. Am J Manag Care. 2022;28(Suppl 4):S60-S68.
9. Danne T, Nimri R, Battelino T, et al. International consensus on use of continuous glucose monitoring. Diabetes Care. 2017;40(12):1631-1640.
10. Shepard JG, Airee A, Dake AW, et al. Limitations of A1c interpretation. South Med J. 2015;108(12):724-729.11. American Diabetes Association Professional Practice Committee. 7. Diabetes Technology: Standards of Care in Diabetes—2024. Diabetes Care. 2024;47(Suppl 1):S126-S144.
12. Hirsch IB, Miller E. Integrating continuous glucose monitoring into clinical practices and patients’ lives. Diabetes Technol Ther. 2021;23(S3):S72-S80.
13. Introduction. Diabetes Care. 2017;40(Suppl 1):S1-S2.14. Pleus S, Ulbrich S, Zschornack E, et al. Documentation of skin-related issues associated with continuous glucose monitoring use in the scientific literature. Diabetes Technol Ther. 2019;21:538-545.
15. Maahs DM, DeSalvo D, Pyle L, et al. Effect of acetaminophen on CGM glucose in an outpatient setting. Diabetes Care. 2015;38(10):e158-e159.
16. Ginsberg BH. Factors affecting blood glucose monitoring: sources of errors in measurement. J Diabetes Sci Technol. 2009;3:903-913.17. Unveiling the latest evidence: CGM’s evolving impact on diabetes care. May 2024. Am J Manag Care. https://cdn.sanity.io/files/0vv8moc6/ajmc/75a26bbcac876d72ce684d37e0bb462c22c73d80.pdf/AJP1286%20Dexcom%20Report.pdf.pdf. Accessed August 14, 2024.
18. Centers for Medicaid & Medicare Services. Glucose monitor—policy article. 2023. www.cms.gov/medicare-coverage-database/view/article.aspx?articleid=52464&contractorName=all&sortBy=updated&bc=13l.
19. ElSayed NA, Aleppo G, Aroda VR, et al. 7. Diabetes technology: Standards of care in diabetes–2023. Diabetes Care. 2023;46(suppl 1):S111-S127.
20. Samson SL, Vellanki P, Blonde L, et al. American Association of Clinical Endocrinology consensus statement: comprehensive type 2 diabetes management algorithm–2023.update. Endocr Pract. 2023;29(5):305-340. doi:10.1016/j. eprac.2023.02.001. Accessed October 10, 2024.
The content contained in this article is for informational purposes only. The content is not intended to be a substitute for professional advice. Reliance on any information provided in this article is solely at your own risk.
To comment on this article, contact rdavidson@uspharmacist.com.





