US
Pharm.
2006;6:HS-5-HS-14.
Hepatitis
B virus (HBV) infection is a worldwide concern. An estimated 350 million
people are chronically infected with HBV, a leading cause of chronic
hepatitis, cirrhosis, and hepatocellular carcinoma.1 These
complications of chronic hepatitis B infection lead to approximately 500,000
deaths worldwide annually.2 In the United States, there are an
estimated 1.25 million hepatitis B carriers; of this group, 15% to 40% will
develop serious hepatic complications during their lifetime.3 This
article reviews several aspects of chronic hepatitis B, including pathogenesis
of HBV, available treatment options, and implications for antiviral therapy.
Epidemiology
Although individuals with chronic
hepatitis B infection live in all parts of the world, HBV is especially
endemic in such areas as southeast Asia, China, and Africa, where more than
half of the population is infected at some time in their lives and more than
8% are chronic carriers of the virus.1 In such high-prevalence
areas, most infections are acquired at birth as a result of perinatal
transmission or during early childhood as a result of transmission through
open cuts and sores from one child to another. Areas with low prevalence of
hepatitis B infection include North America, western Europe, and Australia,
where the infections occur as a result of horizontal transmission among young
adults.1 HBV infections in developed countries usually result from
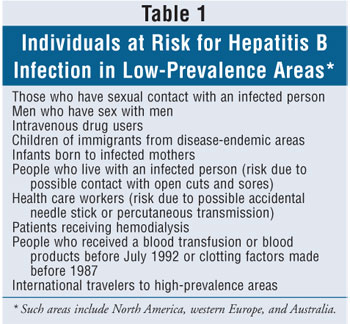
sexual activity, intravenous (IV) drug use, or occupational exposure. Other
risk factors for acquiring HBV in low-prevalence areas, such as the U.S., are
listed in table 1. HBV can survive outside the body for a prolonged period of
time, and carriers of the virus can shed large quantities of viral particles
through open cuts or sores.3 Since HBV is present in serum in large
quantities, the virus can also be detected in semen, saliva, and cervical
secretions. The risk of developing chronic HBV infection after HBV exposure
ranges from 90% in infants and children, possibly due to their immature immune
system, to less than 10% in immunocompetent adults. Immunocompromised adults
are likewise at higher risk for developing chronic HBV infection after acute
exposure.
Pathophysiology
Virology:
HBV is a partially double-stranded DNA virus belonging to the Hepadnaviridae
family.2 Hepatitis B surface antigen (HBsAg) coats the surface of
HBV and is the major viral protein in both acute and chronic infection, as
well as in chronic carriers. Hepatitis B core antigen (HBcAg) is the
nucleocapsid that encloses viral DNA. The immune response crucial for killing
infected hepatocytes requires HBcAg-derived peptides to be expressed on the
surface of the liver cells. Hepatitis B e antigen (HBeAg) is a circulating
peptide derived from the core gene antigen and exported from the hepatocytes.
HBeAg is a marker of active viral replication, and therefore, is present only
in individuals with circulating serum HBV DNA. The understanding of the HBV
life cycle has important implications for drug therapy of chronic hepatitis B
infection. The HBV polymerase is both an endogenous DNA polymerase and a
reverse transcriptase involved in the synthesis of negative DNA strands from
genomic RNA. Therefore, several reverse transcriptase inhibitors are currently
utilized for treatment and prevention of chronic hepatitis B.
Transmission and clinical features:
HBV is transmitted by the exposure of mucous and percutaneous membranes
(e.g., through accidental needle stick) to infectious blood and body fluids.
1 Common routes of exposure are sexual intercourse, IV drug abuse, and
perinatal transmission from infected mother to child. After HBV exposure, the
incubation period is long and variable, ranging from 45 to 160 days. In most
circumstances, HBV is not cytotoxic (i.e., it does not kill liver cells). It
is the host immune response to the presence of HBV antigen that leads to the
destruction of the liver cells and subsequent liver disease.1,2
Clinical signs and symptoms of acute HBV infection occur more often in adults
than in infants or children. Despite active viral replication, less than 10%
of children and only 30% to 50% of adults with acute HBV infections will have
symptomatic disease. Most acute HBV infections in adults result in complete
recovery. Only 1% of patients with acute HBV infection develop fulminant
hepatic failure, and an average of 5% of adults with acute HBV infection
develop chronic infections.1
The clinical presentations associated with
chronic HBV encompass a wide spectrum of signs and symptoms, ranging from an
asymptomatic carrier state to chronic hepatitis, in which patients experience
complications associated with liver cirrhosis or hepatocellular carcinoma.
Chronic hepatitis may progress to end-stage liver disease in approximately 15%
to 40% of patients, and the risk of hepatocellular carcinoma in patients with
chronic hepatitis B is 100 times higher than that of noncarriers.4
In patients with chronic hepatitis B whose condition has progressed to
end-stage liver disease, chronic occurrence of jaundice and enlarged liver
with elevated alanine aminotransferase (ALT) levels are typical features of
the disease. Chronic hepatitis B involves an early replicative phase with
active liver disease and a late phase with low or undetectable levels of viral
replication and remission of liver disease. The four types of chronic
hepatitis B infection are described in table 2.

Diagnosis and serology:
Chronic hepatitis B infection is
defined as hepatic inflammation due to the presence of HBV.3
Chronic infections are divided into HBeAg-positive and HBeAg-negative
subgroups. Diagnosis is made when a positive detection of HBsAg is made in a
patient on at least two occasions in a six-month period, with the presence of
HBV DNA with serum levels greater than 100,000 copies per mL.3
Liver enzyme levels are either persistently or intermittently elevated over
periods of six months or longer. Inactive carrier state is defined as
persistent HBV infection without inflammation of the liver.3
Patients determined to be in an inactive carrier state are HBsAg-positive for
greater than six months, HBeAg-negative, and HBeAg antibody-positive
(anti-HBe). They also have undetectable serum HBV DNA and normal liver enzyme
levels.
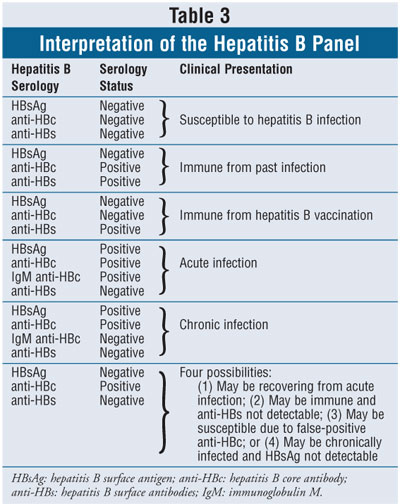
HBsAg can be detected from as early as week
1 after an acute infection and may remain present until resolution of the
infection.2 Detection of HBsAg indicates that a person is
infectious. Shortly after the presence of HBsAg, antibodies to hepatitis B
surface antigen (anti-HBs) can develop and persist for years and confer
immunity if a person is reinfected. HBeAg is a marker of viral replication
detectable early in the acute phase that persists in chronic HBV infection.
However, 20% to 30% of patients in an inactive carrier state (HBeAg-negative
and anti-HBe-positive) experience reactivation of HBV replication and have
elevated ALT and high HBV DNA levels, known as HBeAg-negative chronic
hepatitis.5 Most of these patients harbor HBV variants in the
precore or core promoter region, which abolish or decrease the production of
HBeAg.3 The presence of both HBsAg and HBeAg suggests high levels
of viral replication. HBcAg causes the development of hepatitis B core
antibodies (anti-HBc), which appear one to two weeks after the presence of
HBsAg. HBcAg does not freely circulate in the blood and therefore is not
measured. Instead, the detection of immunoglobulin M anti-HBc is the most
sensitive diagnostic test for acute HBV infection. During the recovery phase
of the infection, immunoglobulin G is the predominant form of anti-HBc. Since
anti-HBc does not develop in patients immunized against HBV, the detection of
this antibody can distinguish infection from vaccination. Interpretation of
the hepatitis B panel is found in table 3.
Treatment
Despite major progress in the
last decade, available therapeutic options for chronic hepatitis B have
limited long-term efficacy. Careful consideration of a patient's age, severity
of liver disease, and likelihood of response is crucial in treatment success.
Current treatment guidelines6 recommend treating patients with
elevated ALT and high HBV DNA levels, whereas patients who are HBeAg-negative
and have low HBV DNA and persistently normal ALT levels for more than six
months are considered inactive carriers and are rarely treated. There is a 20%
to 30% likelihood of HBV reactivation in inactive carriers during long-term
follow-up. Treatment is recommended in patients who are HBeAg-negative and
anti-HBe–positive and have elevated ALT levels and high HBV DNA detected in
serum. In addition, treatment is not recommended for HBeAg-positive patients
with high HBV DNA levels but normal ALT and minimal liver inflammation found
on liver biopsy; in these patients, the likelihood of treatment-related
seroconversion from HBeAg to anti-HBe is similar to that of patients not
receiving treatment.5
The goals of therapy for chronic hepatitis B
are to achieve sustained suppression of HBV replication and remission of liver
disease, leading to prevention of cirrhosis, liver failure, and hepatocellular
carcinoma.5 The treatment response is assessed through
normalization of ALT level, undetectable HBV DNA, loss of HBeAg, and
improvement of liver histology. Complete response is defined as the decline of
liver enzymes to normal range, seroconversion of HBeAg to anti-HBe, virologic
response, and loss of HBsAg. For HBeAg-negative patients in whom
seroconversion from HBeAg to anti-HBe cannot be used as an end point to assess
response to therapy, undetectable serum HBV DNA and normalization of ALT level
are used to define response. There are four FDA-approved antiviral drugs for
the treatment of chronic hepatitis B infection--interferon (IFN), lamivudine,
adefovir, and entecavir (table 4). In addition, there are several promising
new therapeutic options currently in clinical trials. In general, high
pretreatment ALT level is the most consistent factor associated with increased
likelihood of seroconversion for drug therapy. Although indications for
treatment are largely based on ALT levels, histologic assessment in patients
with normal or mildly elevated ALT levels also has an important role in
treatment decisions, as many of these patients show evidence of advanced
hepatic disease on liver biopsy.5

Interferon:
IFN is an endogenous cytokine that induces the immune system to exert
antiviral activity. Various recombinant forms are currently available for the
treatment of chronic hepatitis B. IFN was the first effective therapeutic
option in both HBeAg-positive and HBeAg-negative chronic hepatitis B.7
In addition, IFN is the only medication that has been proven to reduce and
eliminate HBV infection in patients with chronic hepatitis B and has not been
associated with viral resistance.8,9 IFN alfa-2b (Intron-A) has
been approved for the treatment of chronic HBV infection throughout the world,
including adults and children in the U.S., whereas IFN alfa-2a (Roferon) and
IFN alfacon-1 (Infergen) are not approved for this indication, although these
drugs may also be used to treat HBV infection.10 The dosing for the
different forms of conventional IFN ranges from 15 million to 35 million units
per week, and dosing for new pegylated forms of IFN alfa-2a (Pegasys) is 180
mcg weekly. Conventional IFN is administered subcutaneously or intramuscularly
daily or three times per week, and pegylated forms are administered once
weekly. The most common side effects of IFN include flu-like symptoms,
fatigue, irritability, depression, and bone marrow suppression.11
In addition to low HBV DNA concentration of 200 pg/mL, high pretreatment ALT
level is also a reliable predictor of a positive response to IFN. Liver injury
and elevated ALT concentrations are due to active host immune response to
viral infection, and IFN requires cell-mediated immune responses to exert
antiviral immunoregulatory activities by augmenting the host immune system.
Early controlled studies showed that a four-
to six-month course of IFN alfa achieved seroconversion from HBeAg to anti-HBe
in 33% of patients with chronic hepatitis B, compared to 12% of untreated
controls.12 The responses after IFN alfa, including normalization
of ALT, appeared to be durable in the majority of these patients. IFN alfa
treatment of chronic HBV has also been reported to reduce the risk of
hepatocellular carcinoma.13 Pegasys and PEG-Intron (IFN alfa-2b),
pegylated forms of IFN, have been used in phase III trials, and Pegasys is now
FDA approved for chronic hepatitis B. A large phase III trial of pegylated
alfa-2a in HBeAg-negative patients showed a significant rate of sustained
viral suppression that was better than lamivudine alone but was not improved
by the addition of lamivudine.9 The factors predictive of sustained
response to IFN alfa are low pretreatment HBV DNA levels, high ALT levels, and
active liver disease demonstrated by liver biopsy.3 High
pretreatment ALT level appears to be the most important factor associated with
sustained response.5
The major drawback of IFN alfa is the side
effects from which many patients experience difficulty with the therapy (table
5). Approximately one third of patients treated with IFN alfa may require dose
reduction, and up to 5% may discontinue the therapy due to adverse events.
14 The adverse events associated with the drug also lead to poor drug
adherence and intermittent discontinuation. Up to 40% of patients treated with
IFN reportedly developed IFN-induced depression and other psychological
disturbances. Depressive symptoms, anxiety, and irritability may persist
throughout the course of IFN treatment and for a few weeks after cessation of
therapy.3 Patients with advanced cirrhosis usually have leukopenia
and thrombocytopenia that can be exacerbated by the drug. In addition, many
patients experience flare of liver disease during the administration of IFN
alfa, and though this may be a marker of enhanced responsiveness to the
therapy, many patients do not tolerate this drug-induced exacerbation of liver
disease.2 Treatment with IFN is generally contraindicated in
decompensated liver disease, since flares in these cases may precipitate overt
liver failure.2 Other contraindications for IFN therapy include
ongoing alcohol or drug abuse and autoimmune disease.

Lamivudine:
Lamivudine, introduced for the treatment of chronic hepatitis B infection in
the late 1990s, is a nucleoside analogue with potent activity against HBV.
Lamivudine exerts its antiviral activity by inhibiting viral DNA polymerase
activity. Lamivudine is a safe drug with rare and usually mild side effects.
Dosing adjustment is recommended in patients with renal impairment. In
general, treatment with lamivudine at a daily oral dose of 100 mg results in
normalization of aminotransferase in about 70% of patients, about a 90%
decrease in baseline HBV DNA, and about a 15% higher rate of seroconversion
compared to placebo.15 HBeAg seroconversion after the cessation of
lamivudine has been reported in 70% to 90% of patients in Western countries
and 38% to 83% of patients from southeast Asia.16,17 In addition,
significant reduction of progression of the liver disease, as shown in biopsy,
was shown in lamivudine-treated patients compared to placebo.
Advantages of lamivudine therapy include its
low side-effect profile and its relatively low cost. The main disadvantage of
lamivudine is the frequent emergence of viral resistance due to mutations
within the tyrosine-methionine-aspartate-aspartate (YMDD) motif of the HBV
polymerase gene.18 Clinical presentation of viral resistance is
expressed by the reappearance of serum HBV DNA after an initial clearance of
viremia. This virologic breakthrough usually develops after the first six
months of lamivudine monotherapy, and the rate of resistance increases
progressively throughout the continued therapy, ranging from 15% to 30% at 12
months to over 50% after three years and over 70% after five years.19-22
Moreover, the emergence of this resistance has been shown to be associated
with a lower likelihood of seroconversion, and eventually, a negative impact
on liver histology. Another disadvantage of lamivudine therapy is that optimal
duration of therapy is unknown.23 A three-year course of effective
lamivudine therapy resulted in virologic and biochemical relapses in the
majority of HBeAg-negative patients.10 The disadvantage of all oral
agents for HBV infection, including lamivudine, is low rate of durability of
response after the cessation of therapy, which occurs at less than 50%,
especially in HBeAg-negative patients treated with lamivudine who relapse
after discontinuation of therapy in almost all cases.
Adefovir:
Adefovir is a nucleotide analogue approved for the treatment of
HBeAg-negative and HBeAg-positive liver disease, including the
lamivudine-resistant mutant. Adefovir is an oral prodrug that undergoes two
intracellular phosphorylations to become an active moiety and also elicits its
antiviral activity by inhibiting the viral polymerase. Adefovir was initially
developed as a reverse transcriptase inhibitor of HIV but was found to be
nephrotoxic at the dosage required for inhibition of HIV replication.2
In lower doses (10 mg daily), however, adefovir was shown to be potent
against HBV, including the lamivudine-resistant HBV, with low a rate of
nephrotoxicity. Although renal toxicity due to adefovir is rare, monitoring of
creatinine is recommended with adefovir therapy, especially with
coadministration of nephrotoxic medications. Dosing adjustments are also
recommended for patients with reduced renal function and for those requiring
hemodialysis.
In clinical trials, patients treated with
adefovir 10 mg daily experienced significantly greater reductions in HBV DNA
and ALT compared to placebo. Adefovir was also found to be effective in
patients who had not responded to lamivudine therapy.24 In this
study, 67% of patients treated with adefovir had improvements in liver
histology and no worsening of fibrosis, compared to 19% of patients on
placebo. Adefovir was generally well tolerated and had a safety profile
similar to placebo. In long-term therapy with adefovir in HBeAg-negative
patients, benefits were maintained in patients treated for 144 weeks with
infrequent emergence of viral resistance.25 To date, resistance has
been seen in 5.9% of patients at three years and 18% at four years.
Combination therapy with lamivudine does not appear to be beneficial in
treatment-naïve patients or those with lamivudine resistance.
Entecavir:
Entecavir is a nucleoside analogue with potent activity against HBV. It
inhibits HBV replication at three different points: priming of HBV DNA,
reverse transcriptase of the negative DNA strand from genomic RNA, and
synthesis of the positive strand of HBV DNA.26 Entecavir is an oral
drug that is FDA-approved for the treatment of HBV, with dosing at 0.5 mg
daily in treatment-naïve patients and 1 mg daily in lamivudine-treated
patients. Entecavir is generally well tolerated with a low side-effect
profile. Dose adjustment is recommended in patients with renal dysfunction.
In a 96-week study comparing entecavir with
lamivudine in HBeAg-positive chronic hepatitis B patients, 82% of
entecavir-treated patients maintained their response 24 weeks after
discontinuation of treatment, compared with 72% of the lamivudine-treated
patients.27 At 96 weeks of treatment, 80% of the entecavir group
had undetectable HBV DNA compared with 39% of the lamivudine group. In a
two-year resistance study reported for entecavir, the development of entecavir
resistance was shown to require preexisting lamivudine resistance substitution.
28 The one-year entecavir treatment showed no resistance in
nucleoside-naïve patients and 1% resistance in patients with lamivudine
resistance prior to treatment. By two years of entecavir treatment, 10% of
patients with prior lamivudine resistance developed resistance to entecavir,
but none of the treatment-naïve patients showed evidence of entecavir
resistance.
New emerging therapies for chronic hepatitis
B infection: Approved treatments for chronic hepatitis B are limited by low
rates of sustained response, toxicities, or resistance. There are several new
agents currently under development for the management of chronic HBV to
address the need for more potent antiviral effects, fewer adverse events, and
minimal or no risk for drug resistance. Clevudine is a nucleoside analogue
with a long half-life currently in phase III clinical trials for chronic
hepatitis B infection.29 Reported findings from these trials
demonstrated that clevudine is a potent inhibitor of HBV in both
HBeAg-positive and HBeAg-negative patients.30,31 However, in
vitro studies suggest that clevudine is ineffective against
lamivudine-resistant HBV.
Telbivudine is another nucleoside analogue
currently in phase III clinical trials for treatment of chronic hepatitis B.
Data from a 76-week study showed greater potency with telbivudine compared to
lamivudine, but telbivudine did not confer activity against
lamivudine-resistant HBV.32
Tenofovir is a reverse transcriptase
inhibitor effective against HBV, including the lamivudine-resistant HBV.29
Recently published data suggest that tenofovir may be more potent than
adefovir.33
Liver transplantation:
For patients with end-stage liver disease due to chronic hepatitis B
infection, transplantation is an option. To prevent reinfection of
transplanted liver, hepatitis B immune globulin (HBIG) is administered
immediately after transplantation, which decreases the long-term reinfection
rate by half and extends the two-year survival rate from 50% to 80%. The
addition of lamivudine to HBIG further reduces the reinfection rate to about
10% and increases the five-year rate of infection-free survival to
approximately 80%. HBIG, however, is very expensive and substantially
increases the cost of liver transplantation in patients with chronic hepatitis
B infection.
Role of the Pharmacist
Chronic hepatitis B infection
continues to pose a serious public health problem. Several therapeutic options
are currently available for the treatment of chronic HBV, and new emerging
treatments for HBV continue to be studied to further advance the therapeutic
outcome of the disease. Pharmacists in direct care of patients with chronic
HBV should be monitoring for appropriateness of drug therapy and adverse
events associated with drug treatment. Especially for patients on IFN alfa
therapy, monitoring of lab values and observation for other side effects are
crucial in drug adherence and treatment success. Pharmacists should counsel
patients with chronic hepatitis B infection on immunization, avoiding
hepatotoxic drugs and dietary supplements, including alcohol and herbal
medications, and prevention of HBV transmission, including avoiding high-risk
sexual activities and IV drug abuse. Patients who have been newly diagnosed
with chronic hepatitis B should be encouraged to be tested for HIV, as this
may have an important implication on drug therapy and viral resistance.
Appropriate dose adjustment recommendations should be made when necessary, and
drug information on other therapeutic options should be readily available by
pharmacists. Pharmacists who are in direct contact with select population
groups that should be screened for HBV infection (e.g., persons born in
endemic areas and IV drug users) should be diligent in providing information
on the disease and therapeutic options available.
REFERENCES
1. Lee WM. Hepatitis B virus
infection. N Engl J Med. 1997;337:1733-1745.
2. Ganem D, Prince AM. Hepatitis B
virus infection--natural history and clinical consequences. N Engl J
Med. 2004;350:1118-1129.
3. Lok AS, McMahon BJ. Chronic
hepatitis B. Hepatology. 2001;34:1225-1241.
4. Beasley RP. Hepatitis B virus: the
major etiology of hepatocellular carcinoma. Cancer. 1988;61:1942-1956.
5. Wong SN, Lok AS. Treatment of
hepatitis B: who, when, and how? Arch Intern Med. 2006;166:9-12.
6. Lok AS, McMahon BJ. Chronic
hepatitis B: update of recommendations. Hepatology. 2004;39:857-861.
7. Hoofnagle JH, di Bisceglie AM. The
treatment of chronic viral hepatitis. N Engl J Med. 1997;336:347-356.
8. di Bisceglie AM. Long-term outcome
of interferon-alpha therapy for chronic hepatitis B. J Hepatology.
1995;22(suppl 1):65-67.
9. Marcellin P, Lau GK, Bonino F, et
al. Peginterferon alfa-2a alone, lamivudine alone, and the two in combination
in patients with HBeAg-negative chronic hepatitis B. N Engl J Med.
2004;351:1206-1217.
10. Papatheodoridis GV, Hadziyannis
SJ. Review article: current management of chronic hepatitis B. Aliment
Pharmacol Ther. 2004;19:25-37
11. Vinayek R, Shakil O. Adverse
events associated with interferon-alfa therapy in patients with chronic viral
hepatitis. Viral Hep Rev. 1997;3:167-177.
12. Wong DK, Cheung AM, O'Rourke K,
et al. Effect of alpha-interferon in patients with hepatitis B e
antigen-positive chronic hepatitis B: a meta-analysis. Ann Intern Med.
1993;119:312-323.
13. Ikeda K, Saitoh S, Suzuki Y, et
al. Interferon decreases hepatocellular carcinogenesis in patients with
cirrhosis caused by the hepatitis B virus; a pilot study. Cancer.
1998;82:827-835.
14. Wong JB, Koff RS, Tine F, Pauker
SG. Cost-effectiveness of interferon-alpha 2b treatment for hepatitis B e
antigen-positive chronic hepatitis B. Ann Intern Med. 1995;122:664-675.
15. Dienstag JL, Schiff ER, Wright
TL, et al. Lamivudine as initial treatment for chronic hepatitis B in the
United States. N Engl J Med. 1999;341:1256-1263.
16. Schiff E, Cianciara J, Karayalcin
S, et al. Durable HBeAg and HBsAg seroconversion after lamivudine for chronic
hepatitis B. J Hepatol. 2000;32(suppl 2):99.
17. Chang TT, Lai CL, Liaw YF, et al.
Incremental increases in HbeAg seroconversion and continued ALT normalization
in Asian chronic HBV (CHB) patients treated with lamivudine for four years.
Antiviral Ther. 2000;5(suppl 1):44.
18. Allen MI, Deslauriers M, Andrews
CW, et al. Identification and characterization of mutations in hepatitis B
virus resistant to lamivudine. Lamivudine Clinical Investigation Group.
Hepatology. 1998;27:1670-1677.
19. Chayama K, Suzuki Y, Kobayashi M,
et al. Emergence and takeover of YMDD motif mutant hepatitis B virus during
long-term lamivudine therapy and re-takeover by wild type after cessation of
therapy. Hepatology. 1998;27:1711-1716.
20. Lau DT, Khokhar MF, Doo E, et al.
Long-term therapy of chronic hepatitis B with lamivudine. Hepatology.
2000;32:828-834.
21. Papatheodoridis GV, Dimou E,
Laras A, et al. Course of virologic breakthroughs under long-term lamivudine
in HBeAg-negative precore mutant HBV liver disease. Hepatology.
2002;36:219-226.
22. Lai CL, Dienstag J, Schiff E, et
al. Prevalence and clinical correlates of YMDD variants during lamivudine
therapy of patients with chronic hepatitis B. Clin Infect Dis.
2003;36:687-696.
23. Hadziyannis SJ, Papatheodoridis
GV, Vassilopoulos D. Treatment of HBeAg-negative chronic hepatitis B. Semin
Liver Dis. 2003;23:81-88.
24. Marcellin P, Chang TT, Lim SG, et
al. Adefovir dipivoxil for the treatment of hepatitis B e antigen-positive
chronic hepatitis B. N Engl J Med. 2003;348:808-816.
25. Hadziyannis SJ, Tassopoulos NC,
Heathcote EJ, et al. Long-term therapy with adefovir dipivoxil for
HbeAg-negative chronic hepatitis B. N Engl J Med. 2005;352:2673-2681.
26. Gish RG. Current treatment and
future directions in the management of chronic hepatitis B viral infection.
Clin Liver Dis. 2005;9:541-565.
27. Gish RG, Chang TT, Gadano A, et
al. Entecavir results in substantial virologic and biochemical improvement and
HbeAg seroconversion through 96 weeks of treatment in HbeAg-positive chronic
hepatitis B patients. Hepatology. 2005;42:267A.
28. Colonno R, Rose R, Levine S, et
al. Entecavir two year resistance update: no resistance observed in nucleoside
naive patients and low frequency resistance emergence in lamivudine refractory
patients. Hepatology. 2005;42:573A.
29. Lok AS. New treatment of chronic
hepatitis B. Semin Liver Dis. 2004;24(suppl 1):77-82.
30. Yoo BC, Kim JH, Lee KS, et al. A
24-week clevudine monotherapy produced profound on-treatment viral
suppressions as well as sustained viral suppression and normalization of
aminotransferase levels for 24 weeks off-treatment in HbeAg-positive chronic
hepatitis B patients (abstract). Hepatology. 2005;42:270A.
31. Yoo BC, Chung UH, Han BH, et al.
Clevudine is highly efficacious in HBeAg-negative chronic hepatitis B patients
with a sustained antiviral effect after cessation of therapy (abstract).
Hepatology. 2005;42:268A.
32. Lai CL, Gane E, Liaw YF, et al.
Telbivudine (LdT) vs. lamivudine for chronic hepatitis B: first-year results
from the international phase III GLOBE trial (abstract). Hepatology.
2005;42:748A.
33. van Bommel F, Wunsche T, Mauss S,
et al. Comparison of adefovir and tenofovir in the treatment of
lamivudine-resistant hepatitis B virus infection (abstract). Hepatology
. 2004;40:1421-1425.
To comment on this article,
contact
editor@uspharmacist.com.






