One of the greatest challenges of clinical medicine is the management of pain.1 The unpleasant sensation that defines pain is due to complex neurochemical processes in both the peripheral and central nervous system (CNS).1 Due to its subjective nature, the treating clinician must depend upon the patient’s unique perception and description of pain.1 Self-reports of pain provide the most accurate and reliable evidence of pain presence and intensity, according to the American Geriatrics Society.2 Pain can also be dynamic, causing fluctuations not only in the intensity, but in the type of pain (e.g., nociceptive, neuropathic, inflammatory), as well. Alleviation of pain depends on its type.
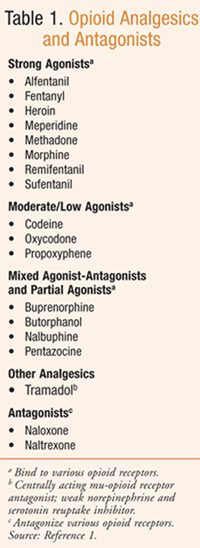
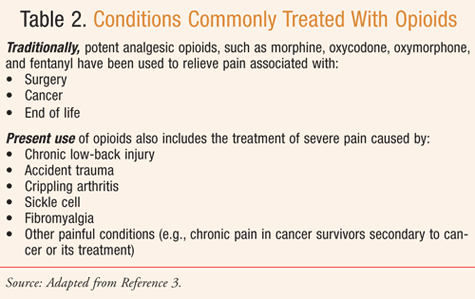
Opioids, natural or synthetic compounds that produce morphine-like effects (TABLE 1), have traditionally been the mainstay for the treatment of severe pain associated with surgery, cancer, and end of life.1,3 The use of these potent analgesics, such as morphine, oxycodone, oxymorphone, and fentanyl, has evolved over time to include a broader variety of conditions causing severe pain, including those associated with injury or disease (TABLE 2). However, opioids are associated with potentially serious results, including opioid-related adverse effects and serious adverse outcomes associated with abuse—thus, the challenge to health care providers in a variety of disciplines, including pharmacy: to balance the risk versus benefit.3,4
The American Pain Society (APS) combined forces with the American Academy of Pain Medicine (AAPM) in developing an evidence-based clinical practice guideline to assist clinicians in both primary care and specialty settings in the management of chronic opioid therapy in patients with chronic noncancer pain.3 A systematic review of the evidence on chronic opioid therapy for chronic noncancer pain was conducted; then, a multidisciplinary panel of pain-management experts reviewed the evidence to formulate the guideline.3,4
The expert panel concluded that chronic opioid therapy can be an effective therapy for carefully selected and monitored patients with chronic noncancer pain. The benefits need to be weighed against the risk of potential harm, including opioid-related adverse effects and outcomes related to the abuse potential of opioids.3 Tolerance or dependence may result from extended use; health care providers, including pharmacists, should be alert to problems of abuse, misuse, and diversion.5 The APS’s peer-reviewed Journal of Pain (www.jpain.org) published the clinical guideline in October 2009; pharmacists are encouraged to review the document in its entirety (see Reference 3).
Chou et al made recommendations in the final document, providing specific guidance regarding the following categories: patient selection and risk stratification, informed consent, medication management plans, initiation and titration of therapy, methadone, patient monitoring, dose escalations, high-dose therapy, rotation of opioids, discontinuation of therapy, adverse effects, adjunct psychotherapeutic interventions, safety during work and while driving, identifying a medical home, when it is appropriate to obtain a consultation, breakthrough pain, opioids during pregnancy, and policies regarding opioid therapy.3
The treatment of the following conditions is outside the scope of the new guideline: acute pain, postsurgical pain, labor pain, cancer pain, pain at end of life, or chronic noncancer pain in pediatric or adolescent patients; the APS has separate guidelines that address the management of cancer pain (see Reference 6) and sickle cell pain (see Reference 7).3
While the 25 recommendations were not rated as supported by high-quality evidence, and only four recommendations were viewed as supported by even moderate-quality evidence, there was unanimous consensus among the expert panel on almost all of its recommendations.3 The authors indicate that more research is also required regarding how policies governing prescribing and use of chronic opioid therapy affect clinical outcomes.3
Patient Selection and Appropriate Monitoring
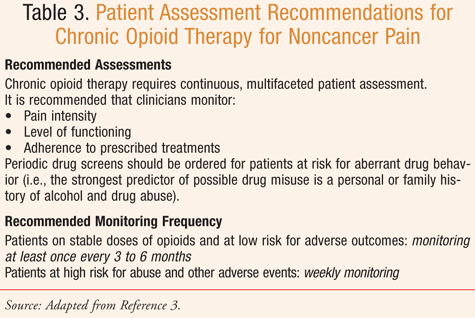
Pharmacists are encouraged to review the screening, assessment, and monitoring tools outlined and available in the guidelines and in the Resources sidebar.3 Chou et al stress the importance of reassessing patients periodically and when changes occur. An example of patient-assessment recommendations for chronic opioid therapy, including monitoring frequency, can be found in TABLE 3. Chou et al indicate that more frequent and intense monitoring is required for patients at high risk for abuse as compared to lower risk patients. While the best methods for managing high-risk patients are not backed by evidence, the guideline recommends using pill counts, uridrug screening, family member or caregiver interviews, and prescription monitoring data to check for possible abuse.3,8

When older adult patients are refractory to nonopioid therapeutic interventions, they may continue to suffer from chronic/persistent pain such as disabling osteoarthritis pain. The new guidelines indicate that, for example, if a 60-year-old patient has no significant psychiatric comorbidities, major medical comorbidities, or personal or family history of drug abuse or addiction, he or she would be assessed as someone with high potential benefits from chronic opioid therapy relative to the potential risks.3 The experts agree that in most clinical settings, chronic opioid therapy could be prescribed to this patient as long as routine monitoring (as outlined in section 5 of the clinical practice guideline) is implemented along with the pharmacologic therapy.3
Pharmacists should note, however, that many seniors have comorbidities with medication regimens consisting of polypharmacy with multiple potential drug–drug and drug–disease interactions. Opioids should be used with caution in the elderly, debilitated, and those with severe hepatic or renal function.5 In patients with hypovolemia, concurrent vasodilating medications, or head injury, hemodynamic effects (e.g., hypotension, orthostasis) may be exaggerated.5 In opioid overdose, an opioid antagonist (e.g., naloxone; TABLE 1) is used to reverse coma and respiratory depression, causing the patient to be revived and alert; with lower dosing, partial reversal is also possible.1 Opioid antagonist therapy (e.g., naltrexone) is also used in the treatment of opioid addiction.1
The concurrent use of agonist/antagonist analgesics (TABLE 1) has the potential to precipitate withdrawal symptoms and/or reduced analgesic efficacy in patients following prolonged therapy with mu-opioid agonists; following their prolonged use, abrupt withdrawal may also lead to withdrawal symptoms.5
Assessing pain may be especially challenging in older adults with sensory impairments, disability, and dementia.2 While seniors are more likely to suffer from arthritis, bone, and joint disorders causing persistent pain, changes in the patient’s condition should not be overlooked.2,9,10 Acute pain may indicate a new condition that is distinguishable from an exacerbation of persistent pain and should be evaluated by a physician.2 When increased motility is achieved through the treatment or reduction of pain intensity, improvement may result not only in physical functioning, but also in psychosocial functioning, allowing for an increase in social engagement.2
Conclusion
Although opioids may be associated with potentially harmful effects, including opioid-related adverse effects and outcomes connected to the abuse potential of opioids, a panel of pain management experts has concluded that chronic opioid therapy can be an effective therapy for carefully selected and monitored patients with chronic noncancer pain. Through expert consensus, a new and comprehensive clinical practice guideline provides recommendations, based on a systematic review of the evidence, to assist clinicians in prescribing potent opioid pain medications for patients with chronic noncancer pain. The authors indicate that more studies are also required to determine how policies that govern prescribing and the use of chronic opioid therapy affect
clinical outcomes.
REFERENCES
1. Howland RD, Mycek MJ. Pharmacology. 3rd ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2006:157-168.
2. The management of persistent pain in older persons. American Geriatrics Society (AGS) Panel on Persistent Pain in Older Adults. 2002. www.americangeriatrics.org/products/positionpapers/JGS5071.pdf. Accessed April 23, 2010.
3. Chou R, Fanciullo GJ, Fine PG, et al. Clinical guidelines for the use of chronic opioid therapy in chronic noncancer pain. J Pain. 2009;10:113-130.e22. www.jpain.org/article/S1526-5900(08)00831-6/fulltext. Accessed April 18, 2010.
4. New guidelines for prescribing opioid pain drugs published. February 10, 2009.
www.physorg.com/news153514787.html.
Accessed April 18, 2010.
5. Semla TP, Beizer JL, Higbee MD. Geriatric Dosage Handbook. 14th ed. Hudson, OH: Lexi-Comp, Inc; 2009.
6. Miaskowski C, Cleary J, Burney R, et al. Guideline for the management of cancer pain in adults and children. Glenview, IL: The American Pain Society; 2005.
7. Benjamin L, Dampier C, Jacox A, et al. Guideline for the management of acute and chronic pain in sickle-cell disease. Glenview, IL: The American Pain Society; 2007.
8. Katz N, Fanciullo GJ. Role of urine toxicology testing in the management of chronic opioid therapy. Clin J Pain. 2002;18:S76–S82.
9. Kane RL, Ouslander JG, Abrass IB. Essentials of Clinical Geriatrics. 5th ed. New York, NY: McGraw-Hill, Inc; 2004:57-70,266-277,129-145,462,511-517
10. Zagaria ME. Consequences of persistent pain. US Pharm. 2008;33(5):28-30.
To comment on this article, contact rdavidson@uspharmacist.com.





