US Pharm. 2020;45(4):48-CV3.
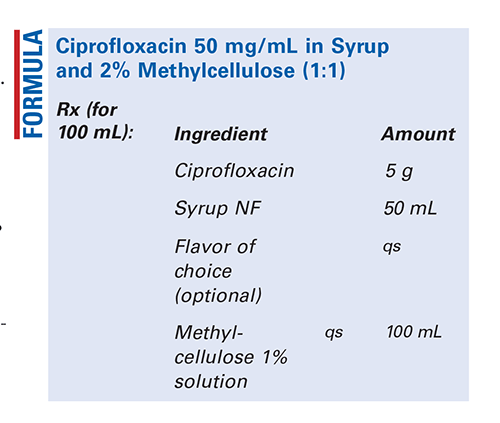
Method of Preparation: Calculate the quantity of each ingredient for the amount to be prepared. Accurately weigh or measure each ingredient. Pulverize the ciprofloxacin tablets or obtain the ciprofloxacin powder. Add a small quantity of syrup and mix to form a smooth, uniform paste. Geometrically, add the remainder of the syrup and mix well. Slowly add the methylcellulose 1% solution, with mixing, until uniform. Package and label.
Use: Ciprofloxacin oral suspension is a fluoroquinolone anti-infective.
Packaging: Package in tight, light-resistant containers.
Labeling: Keep out of reach of children. Keep at room temperature or refrigerated. Shake well. Discard after ____ [time period].
Stability: A beyond-use date of up to 91 days at both refrigerated and room temperature has been used for this compound as prepared.1,2 If the preparation is modified using a flavor or additional ingredients, the USP default beyond-use date or the state board of pharmacy requirements should be used.
Quality Control: Quality-control assessment can include weight/volume, pH, specific gravity, active drug assay, color, rheologic properties/pourability, physical observation, and physical stability (discoloration, foreign materials, gas formation, mold growth).3,4
Discussion: Ciprofloxacin is used to treat infections caused by susceptible bacteria. This compounded preparation is suitable for patients who cannot tolerate preservatives, dyes, certain flavors, and the like, and it can be individualized for specific patient needs.
Ciprofloxacin hydrochloride (HCl) (Ciloxan, Cipro, Proquin, C17H18FN3O3.HCl.H2O, MW 385.82) occurs as faintly yellowish to light-yellow crystals that melt at about 318°C to 320°C. It is sparingly soluble in water (36 mg/mL), slightly soluble in acetic acid, and very slightly soluble in dehydrated alcohol. The pH of a 25 mg/mL aqueous solution is between 3.0 and 4.5. Ciprofloxacin HCl should be stored at room temperature and should be protected from intense ultraviolet light.1,5
Syrup (simple syrup) is a clear, sweet vehicle used as a sweetening agent and as the base for many flavored and medicated syrups. It contains 85% w/v sucrose in water and has a specific gravity of not less than 1.30. Syrup is generally self-preserving as long as the sucrose concentration is maintained sufficiently high. It is best to prepare it without the use of heat, but it may be prepared with boiling water. Syrup should be stored in tight containers, preferably in a cool place.1
Methylcellulose (Methocel) is a practically odorless and tasteless, white to yellowish-white granule or powder that is widely used in oral and topical formulations. It is available in different viscosity grades; the low viscosity grades are used to emulsify oils and as suspending and thickening agents for oral liquids. The pH of a 1% solution is in the range of 5.5 to 8.0. Methylcellulose is hygroscopic and practically insoluble in acetone, ethanol, saturated salt solutions, and hot water, but is it soluble in glacial acetic acid. In cold water, methylcellulose swells and disperses to form a viscous, colloidal dispersion. Its solutions are stable to alkalis and dilute acids at pH values of 3 to 11. Reported incompatibilities include chlorocresol, phenol, resorcinol, tannic acid, silver nitrate, cetylpyridinium chloride, p-aminobenzoic acid, and the parabens. Mineral acid salts, phenols, and tannins coagulate solutions of methylcellulose, but this can be prevented with the addition of 95% ethanol. High concentrations of electrolytes may completely precipitate the dispersion.6
Purified water is water that is obtained by distillation, ion exchange, reverse osmosis, or some other suitable process. Water has a specific gravity of 0.9971 at room temperature, a melting point of 0°C, and a boiling point of 100°C. It is miscible with most polar solvents and is chemically stable in all physical states (ice, liquid, and steam).7
REFERENCES
1. U.S. Pharmacopeia/National Formulary [current revision]. Rockville, MD: U.S. Pharmacopeial Convention, Inc; March 2020.
2. Nahata MC, Morosco RS, Hipple TF, et al. Development of stable oral suspensions of ciprofloxacin. J Appl Ther Res. 2000;3:61-65.
3. Allen LV Jr. Summary of quality-control testing for sterile and nonsterile compounded preparations, part 1: physical and chemical testing. IJPC. 2019;23:211-216.
4. Allen LV Jr. Standard operating procedure for performing physical quality assessment of oral and topical liquids. IJPC. 1999;3:146-147.
5. Allen LV Jr, ed. Remington: The Science and Practice of Pharmacy. 22nd ed. London, England: Pharmaceutical Press; 2012:1339-1340.
6. Allen LV Jr. Methylcellulose. In: Sheskey PJ, Cook WG, Cable CG, eds. Handbook of Pharmaceutical Excipients. 8th ed. London, England: Pharmaceutical Press; 2017:600-604.
7. Dubash D, Shah U. Water. In: Sheskey PJ, Cook WG, Cable CG, eds. Handbook of Pharmaceutical Excipients. 8th ed. London, England: Pharmaceutical Press; 2017:1012-1016.
To comment on this article, contact rdavidson@uspharmacist.com.





