US Pharm. 2024;49(1):14.
Psychedelic drugs, or hallucinogens, are psychoactive substances that change perception, mood, and cognitive function. According to the National Survey on Drug Use and Health, more than 8.1 million people in the United States reported using psychedelics in 2022, an increase of more than 3 million people compared with 2018. Nearly 75% of psychedelic drug use has been reported as recreational; however, an estimated 39% of people have reported use for therapeutic purposes, spurring increased interest in the potential clinical applications of psychedelic drugs for various disorders and diseases.
Rationale: Psychedelics include the classic 5-HT2 agonists (e.g., psilocybin, lysergic acid diethylamide), mixed serotonin-dopamine reuptake inhibitors (e.g., phencyclidine) and releasers or entactogens-empathogens (e.g., 3,4-methylenedioxymethamphetamine [MDMA]), and N-methyl-d-aspartate antagonists (e.g., dextromethorphan, ketamine). Based on these drugs’ mechanisms of action, clinical research has focused on the utility of psychedelics for treating mental-health conditions such as posttraumatic stress disorder (PTSD), treatment-resistant depression (TRD), and substance-use disorders. Recent studies also reveal possible therapeutic effects of psychedelics in preventing and treating brain injury and neurodegenerative diseases (e.g., Alzheimer’s disease, Parkinson’s disease). Key preclinical findings suggest that psychedelics can induce neuroplasticity and synaptogenesis, stimulate neural progenitor cell proliferation, and reduce levels of proinflammatory biomarkers.
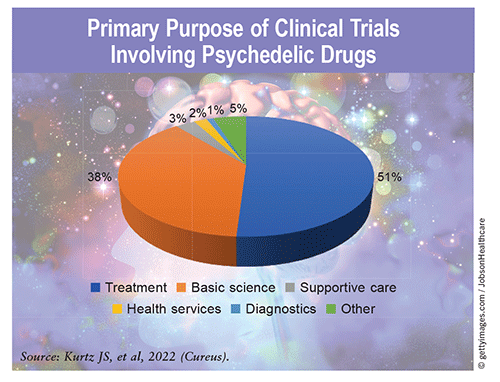
Clinical Trials: The number of clinical trials involving psychedelics has increased globally over the past several years, with 77.1% begun in or after 2017. The majority have been phase I (53.3%) or phase II (25.7%) trials and have primarily studied psychedelics for the treatment of various conditions (51%). Most of these trials have taken place in the U.S. (70.5%) and have largely been sponsored by Yale University (21.9%), Johns Hopkins University (10.5%), and the Multidisciplinary Association for Psychedelic Studies (8.6%). Trial results have been promising to date, and psilocybin and MDMA could be FDA approved for selected disorders by the end of 2024.
Guidance: In the U.S., most psychedelic drugs are designated as Schedule I controlled substances owing to the lack of accepted medical use and/or safety. In 2017, the FDA granted Breakthrough Therapy designations to psilocybin and MDMA for TRD and PTSD, respectively. For greater acceptability of and progress in the potential clinical applications of psychedelic drugs, more rigorous research is needed to elucidate the basic science on a molecular level and assess these drugs’ effectiveness and tolerability. To that end, in June 2023 the FDA Center for Drug Evaluation and Research issued its first draft guidance for sponsors and researchers regarding the development and/or investigation of psychedelic drugs for various medical conditions. This guidance provides considerations for designing clinical trials that are aimed at investigating the efficacy and safety of psychedelic treatments as well as the generalizability to target patient populations.
The content contained in this article is for informational purposes only. The content is not intended to be a substitute for professional advice. Reliance on any information provided in this article is solely at your own risk.
To comment on this article, contact rdavidson@uspharmacist.com.





