US Pharm. 2016;41(3):42-45.
ABSTRACT: Neuropathic neck and back pain often requires a multidisciplinary approach to management in hopes of alleviating the associated economic, humanistic, and societal burdens. While nonpharmacologic and interventional therapies play a role in the treatment of these patients, pharmacologic therapy is often required. Evidence-based recommendations suggest that tricyclic antidepressants, serotonin-norepinephrine reuptake inhibitors, pregabalin, and gabapentin are first-line in neuropathic pain management, while other agents such as lidocaine patches, capsaicin patches, and tramadol may be considered alternative agents. With the many complexities associated with neuropathic pain management, it is essential that practitioners monitor for safety and efficacy of treatment.
Neuropathic pain is a type of chronic pain caused by injury to the nervous system resulting from several different mechanisms, independent of the cause of any disease.1 It affects an estimated 7% to 8% of adults in the general population.2 Chronic low back and neck pain, often related to radiculopathy or sciatica, is a fairly prevalent condition in which 37% of patients have a neuropathic pain component.2 Patients with neuropathic pain experience notable positive (e.g., paresthesias, thermal hyperalgesia, allodynia) and negative (e.g., hypoesthesia, hypoalgesia) symptoms; these symptoms can be severe and may be described as tingling, numbness, burning, shooting, or “electric shocklike.”1,3
There are significant economic, humanistic, and societal burdens associated with neuropathic pain, including higher healthcare costs and lower health-related quality of life, with an increased economic burden attributed to neuropathic back or neck pain.4,5 As this condition is potentially undertreated,6 appropriate assessment and diagnosis are key. The pharmacist can play an active role in patient assessment and referral. Standardized and validated screening tools aid in the assessment process.7-9 Diagnosis is primarily based on history and physical examination and the ruling out of other treatable conditions.9
Clinical Management
There are many complexities associated with the clinical management of neuropathic low back and neck pain.10 Therefore, patients may benefit from a multidisciplinary patient care approach and the use of nonpharmacologic options such as physical or occupational therapy, rehabilitation, support groups, or cognitive behavioral therapy.9 One of the goals of therapy is pain level reduction, and with treatment, patients generally derive a 30% to 50% decrease in pain on a 10-point scale.11 In some cases, interventional therapies may be necessary9,12; however, pharmacologic therapy remains the mainstay of neuropathic pain management.9,11,13
Pharmacologic Agents
Treatment of neuropathic pain often differs from treatment of musculoskeletal back pain, where acetaminophen, nonsteroidal anti-inflammatory drugs (NSAIDs), cyclooxygenase-2 (COX-2) inhibitors, and opioids are commonly indicated. Practice guidelines for neuropathic pain suggest that most drugs used to treat pain have similar efficacy regardless of the type of pain disorder being treated and that recommendations apply to neuropathic pain management in general.11 However, the majority of trials were conducted in patients with diabetic painful polyneuropathy or postherpetic neuralgia (PHN), and studies commonly had a duration of <12 weeks.11,13-15 When selecting drugs, consideration should be taken regarding patient comorbidities and, in some cases, age.
Treatment of neuropathic pain consists of systemic and topical agents (see TABLE 1); adverse drug reactions and clinical considerations are also detailed.1,9,11,13,14,16 Initial treatment is with a single agent, along with a gradual increase in dosage to a target dose. Titration of the dose is usually weekly with an increase that is equal to the initial starting dose and increased thereafter until the target dose is achieved. If the target dose is unsuccessful, then combination therapy is warranted.11 Caution should be taken when combining agents such as tricyclic antidepressants (TCAs), serotonin-norepinephrine reuptake inhibitors (SNRIs), and tramadol in order to avoid serotonin-like adverse events.
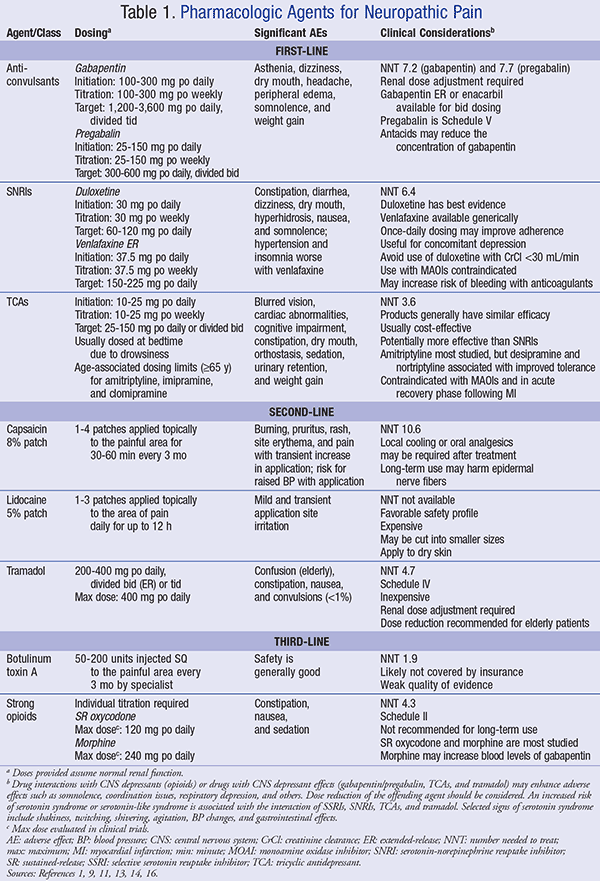
First-Line Agents: These agents are described in TABLE 1. A TCA is the initial preference, specifically nortriptyline or desipramine, because these drugs are better tolerated.11 A TCA is also effective for pain-related insomnia.9 An SNRI is appropriate for a patient with comorbid depression, but is also indicated for those who are not depressed. An anticonvulsant, specifically gabapentin or pregabalin, is also a first-line agent and the drug of choice for patients with a contraindication to a TCA or for a patient with only a partial pain response. TCAs should be used with caution in elderly patients, as they are often associated with cognitive impairment and anticholinergic effects. A combination of first-line agents may also be an alternative. The combination of gabapentin with nortriptyline has been shown to be effective in treatment of diabetic neuropathy; however, no specific combination regimens are currently recommended for neuropathic back or neck pain.9,11
Second-Line Agents: Tramadol is a second-line agent (TABLE 1) that is recommended in place of strong opioids and beneficial for patients with other comorbid pain disorders. Other second-line therapy options consist of topical agents that are legend products and FDA-approved for PHN, but used off-label for other types of neuropathic pain.
When applied to intact skin, a lidocaine 5% patch acts as an anesthetic agent. It is potentially first-line in the elderly or when systemic adverse effects are concerning. OTC concentrations of capsaicin provide only minimal benefit for neuropathic pain, but a topical capsaicin 8% patch is indicated. The patch is applied by a healthcare professional in a medical setting and has special handling, administration, and monitoring procedures.11 The 8% patch is applied to the painful area for a period of 60 minutes and may be repeated every 3 months. The patient must be monitored for an increase in blood pressure due to a transient increase in pain. The skin must be pretreated with a local anesthetic, cleansed with soap and water, and dried before the patch is applied. After the patch is removed, the area should again be cleaned with a cleansing gel that is included in the product packaging. Topical application of ice may be needed after treatment. The used patch and supplies must be disposed of following biomedical waste guidelines.16
Third-Line Agents: Strong opioids, such as morphine, are considered third-line for the management of neuropathic pain (TABLE 1) and can be combined with gabapentin for further relief. However, because of their risk of euphoria, dependence, misuse, and overdose, they are generally considered last-line.11 Another third-line agent is botulinum toxin A. The toxin is subcutaneously injected by a healthcare professional every 3 months. The mechanism of action is thought to inhibit the release of peripheral neurotransmitters and inflammatory mediators from sensory nerves. Use of the toxin remains controversial.17
Although not a routinely recommended therapeutic option, cannabis extract oral spray is approved outside the United States for treatment of muscle spasticity associated with multiple sclerosis and used in FDA clinical trials for cancer pain. The manufacturer is investigating the use of the extract for treatment of neuropathic pain.11,18 Other medications that are generally not recommended for the treatment of neuropathic pain include, but are not limited to, N-methyl-d-aspartate (NMDA) antagonists, valproate, carbamazepine, levetiracetam, and mexiletine. These agents have either inconclusive recommendations or weak/strong recommendations against their use because of negative trials or side effects.11
Emerging Agents: There are notable emerging agents for the management of neuropathic pain. One such agent involves targeting a mutation in the gene SCN9A, which blocks the ability of nerve cells to transmit pain signals, thereby completely eliminating the sensation of pain. The lack of pain sensation is thought to be due to the mutation’s blockage of specific sodium channels in nerve cells known as voltage-gated sodium channels (NaV), specifically NaV1.7. Research is being conducted on drugs that block NaV1.7 sodium channels and the transmission of signals to block pain.19 Additionally, animal studies are being conducted to explore the role of a novel agent associated with nitric oxide production in neuropathic pain.20
Case Study: Neuropathic Low Back Pain
GR is a 58-year-old male seeking advice for treating his chronic back pain. He presents with a complaint of ongoing shooting low back pain, which limits his daily activities, including exercise.
Past Medical History: Hyperlipidemia, hypertension, myocardial infarction, and alcoholism (in recovery since 2000).
Current Medications: Clopidogrel 75 mg daily, benazepril 10 mg daily, atorvastatin 80 mg daily, omeprazole 40 mg daily, trazodone 100 mg bid, tramadol 100 mg qid, meloxicam 15 mg daily, aspirin 81 mg daily, nitroglycerine 0.4 mg prn, and glucosamine 500 mg tid.
Recommendations
The use of trazodone, a serotonin modulator, is not appropriate. One first-line option for treatment of neuropathic pain is a TCA such as nortriptyline.11
Adverse reactions of sedation and cognitive impairment associated with TCAs may in part limit the patient’s exercise activities. Alternatively, duloxetine should be considered, although venlafaxine may be more cost-effective. Venlafaxine may increase blood pressure, and the patient would require monitoring since he has a history of hypertension and is prescribed benazepril. Also, venlafaxine should be given in the morning, rather than at bedtime, to decrease the side effect of insomnia.9
The use of meloxicam, an NSAID, should be assessed. NSAIDs may be used in the self-care treatment of chronic neuropathic pain, but are not effective at times. In addition, an NSAID is contraindicated in this patient because he has a history of cardiovascular disease.
The tramadol dosage is at the maximum daily limit. GR should be assessed for signs of abuse and the dose tapered, not abruptly discontinued. If tramadol is used in combination with nortriptyline, then counseling should be provided regarding the potential for serotonin-like effects with this drug combination.
GR is at risk for bleeding, although he is taking a proton pump inhibitor. Medications that increase the risk of bleeding include clopidogrel, aspirin, trazodone, and meloxicam. The use of glucosamine for neuropathic pain has not been shown to be effective.
Care Plan
Discontinue trazodone and initiate nortriptyline 25 mg daily, increasing by 25 mg weekly to a target dosage of 75 mg daily.16 Discontinue meloxicam. Consider the addition of gabapentin after the target dose of nortriptyline has been reached. Gabapentin is a nonscheduled, first-line drug and, therefore, is more appropriate for this patient. Glucosamine should also be discontinued. An alternative agent such as a lidocaine 5% patch can also be considered.
Conclusion
Overall, there are many complexities associated with neuropathic pain management. An interdisciplinary, integrated approach is necessary to assist patients in achieving a reduction in pain and improvement in quality of life. Pharmacists can contribute to improving outcomes for those living with ongoing neuropathic low back and neck pain by conducting appropriate assessments, providing collaborative medication management, as well as patient education and counseling. Implementation of evidence-based recommendations associated with the use of TCAs, SNRIs, pregabalin, and gabapentin as first-line agents will allow for informed treatment decisions, especially within the primary care setting where most patients with neuropathic pain are managed. Nevertheless, it is still critical to consider patient-specific characteristics and preferences that may impact choice of therapy. Finally, the importance of monitoring patients for safety and efficacy of therapy, as well as ensuring appropriate dosing of agents and drug-interaction prevention, cannot be overemphasized.
REFERENCES
1. Baron R, Binder A, Wasner G. Neuropathic pain: diagnosis, pathophysiological mechanisms, and treatment. Lancet Neurol. 2010;9(8):807-819.
2. International Association for the Study of Pain (IASP). Fact sheets. Epidemiology of neuropathic pain: how common is neuropathic pain, and what is its impact? http://iasp.files.cms-plus.com/AM/Images/GYAP/Epidemiology%20of%20Neuropathic%20Pain.pdf. Accessed September 25, 2015.
3. Dworkin RH, Backonja M, Rowbotham MC, et al. Advances in neuropathic pain: diagnosis, mechanisms, and treatment recommendations. Arch Neurol. 2003;60(11):1524-1534.
4. Doth AH, Hansson PT, Jensen MP, Taylor RS. The burden of neuropathic pain: a systematic review and meta-analysis of health utilities. Pain. 2010;149(2):338-344.
5. Kleinman N, Patel AA, Benson C, et al. Economic burden of back and neck pain: effect of a neuropathic component. Popul Health Manag. 2014;17(4):224-232.
6. Torrance N, Ferguson JA, Afolabi E, et al. Neuropathic pain in the community: more undertreated than refractory? Pain. 2013;154(5):690-699.
7. Haanpää M, Attal N, Backonja M, et al. NeuPSIG guidelines on neuropathic pain assessment. Pain. 2011;152(1):14-27.
8. Mulvey MR, Bennett MI, Liwowsky I, Freynhagen R. The role of screening tools in diagnosing neuropathic pain. Pain Manag. 2014;4(3):233-243.
9. Gilron I, Baron R, Jensen T. Neuropathic pain: principles of diagnosis and treatment. Mayo Clin Proc. 2015;90(4):532-545.
10. Kozma CM, Provenzano DA, Slaton TL, et al. Complexity of pain management among patients with nociceptive or neuropathic neck, back, or osteoarthritis diagnoses. J Manag Care Spec Pharm. 2014;20(5):455-466b.
11. Finnerup NB, Attal N, Haroutounian S, et al. Pharmaco-therapy for neuropathic pain in adults: a systematic review and meta-analysis. Lancet Neurol. 2015;14(2):162-173.
12. Dworkin RH, O’Connor AB, Kent J, et al. Interventional management of neuropathic pain: NeuPSIG recommendations. Pain. 2013;154(11):2249-2261.
13. Moulin DE, Clark AJ, Gilron I, et al. Pharmacological management of chronic neuropathic pain—consensus statement and guidelines from the Canadian Pain Society. Pain Res Manag. 2007;12(1):13-21.
14. Attal N, Cruccu G, Baron R, et al; European Federation of Neurological Societies. EFNS guidelines on the pharmacological treatment of neuropathic pain: 2010 revision. Eur J Neurol. 2010;17(9):1113-e88.
15. Neuropathic Pain: The Pharmacological Management of Neuropathic Pain in Adults in Non-Specialist Settings. NICE Clinical Guidelines, No. 173. London, UK: National Institute for Health and Care Excellence; 2013. www.ncbi.nlm.nih.gov/books/NBK266257. Accessed October 14, 2015.
16. Drug Facts and Comparisons. Facts & Comparisons [online database]. St. Louis, MO: Wolters Kluwer Health, Inc; March 2005. www.wolterskluwercdi.com/facts-comparisons-online/. Accessed October 28, 2015.
17. Oh HM, Chung ME. Botulinum toxin for neuropathic pain: a review of the literature. Toxins. 2015;7(8):3127-3154.
18. GW Pharmaceuticals. Neuropathic pain. www.gwpharm.com/neuropathic-pain.aspx. Accessed October 28, 2015.
19. Waxman SG, Merkies IS, Gerrits MM, et al. Sodium channel genes in pain-related disorders: phenotype–genotype associations and recommendations for clinical use. Lancet Neurol. 2014;13(11):1152-1160.
20. Grodzki AC, Poola B, Pasupuleti N, et al. A novel carboline derivative inhibits nitric oxide formation in macrophages independent of effects on tumor necrosis factor α and inter-leukin-1β expression. J Pharmacol Exp Ther. 2015;352(3): 438-447.
To comment on this article, contact rdavidson@uspharmacist.com.





