US Pharm. 2019;44(10):48-CV.
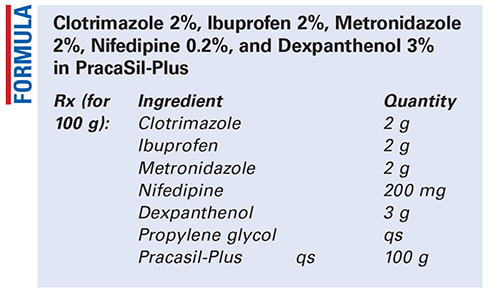
Method of Preparation: Owing to the light sensitivity of metronidazole and nifedipine, work in reduced light and do not expose the materials to light any longer than necessary. Calculate the required quantity of each ingredient for the total amount to be prepared. Accurately weigh and/or measure each ingredient. Pulverize and mix together the clotrimazole, ibuprofen, metronidazole, nifedipine, and dexpanthenol. Levigate this powder with sufficient propylene glycol to make a smooth paste. Incorporate the paste into the PracaSil-Plus and mix until uniform. Package and label.
Use: This preparation may be used in the treatment of complications of the foot that are caused by diabetes.1
Packaging: Package mixture in tight, light-resistant containers.
Labeling: Keep out of reach of children. Use only as directed. For external use. Discard after ____ [time period].
Stability: A beyond-use date of up to 6 months has been used for this anhydrous topical preparation.2
Quality Control: Quality-control assessment can include theoretical weight compared with actual weight, specific gravity, active drug assay, color, texture–surface, texture–spatula spread, appearance, feel, rheologic properties, and physical observations.3,4
Discussion: Clotrimazole (Lotrimin, Mycelex, C22H17ClN2, MW 344.84) occurs as a white to pale yellow, crystalline powder that melts at about 142°C with decomposition. It is freely soluble in alcohol and is practically insoluble in water. Clotrimazole is an antifungal agent used topically and vaginally in the treatment of susceptible fungal infections.5
Ibuprofen (C13H18O2, MW 206.28) is a nonsteroidal anti-inflammatory agent with analgesic and antipyretic activity. It is used in the treatment of inflammatory diseases (rheumatoid arthritis, juvenile arthritis, and osteoarthritis) and pericarditis, as well as for relief of mild-to-moderate pain. It occurs as a white to off-white, crystalline powder with a slight, characteristic odor. Ibuprofen is practically insoluble in water but is highly soluble in alcohol and acetone.1
Metronidazole (C6H9N3O3, MW 171.15) occurs as white to pale yellow, odorless crystals or crystalline powder. It is stable in air but darkens on exposure to light. Metronidazole is sparingly soluble in water and alcohol but is only slightly soluble in ether and chloroform. It is used as an antibiotic.1
Nifedipine (Adalat, Procardia, C17H18N2O6, MW 346.33) is a 1,4-dihydropyridine-derivative calcium-channel blocking agent that occurs as a yellow powder that is affected by exposure to light. It is practically insoluble in water and soluble in alcohol.1,6
Dexpanthenol (C9H19NO4, MW 205.25) occurs as a clear, viscous, somewhat hygroscopic liquid with a slight, characteristic odor. It is freely soluble in water, alcohol, and propylene glycol and is slightly soluble in glycerin. Some crystallization may occur upon standing. Dexpanthenol should be stored in airtight containers. This agent has been used topically in 2% to 5% concentrations in the treatment of various minor skin disorders.7
Propylene glycol (C3H8O2) occurs as a clear, colorless, viscous, practically odorless liquid with a sweet taste that somewhat resembles glycerin. It has a specific gravity of 1.038 g/mL and is miscible with acetone, chloroform, 95% ethanol, glycerin, and water. Propylene glycol is not miscible with fixed oils or light mineral oil; however, it will dissolve some essential oils.8
PracaSil-Plus is a proprietary blend of ingredients—including silicones—in a semipermeable polymer network and pracaxi oil, which contains fatty acids and lipids. It occurs as a pale green, opaque gel with a characteristic odor and has a specific gravity of 0.9 to 1.1 and a viscosity of 14,562 cPs to 51,370 cPs.9
REFERENCES
1. Agbi KE, Carvalho M, Phan H, Tuma C. Case report: diabetic foot ulcer infection treated with topical compounded medications. IJPC. 2017;21(1):22-27.
2. U.S. Pharmacopeia/National Formulary [current revision]. Rockville, MD: U.S. Pharmacopeial Convention, Inc; September 2019.
3. Allen LV Jr. Summary of quality-control testing for sterile and nonsterile compounded preparations, part 1: physical and chemical testing. IJPC. 2019;23(3):211-216.
4. Allen LV Jr. Summary of quality-control testing for sterile and nonsterile compounded preparations, part 2: microbiological testing. IJPC. 2019;23(4):299-303.
5. MedlinePlus. Clotrimazole vaginal. https://medlineplus.gov/druginfo/meds/a682753.html. Accessed September 9, 2019.
6. McEvoy GK, ed. AHFS Drug Information 2016. Bethesda, MD: American Society of Health-System Pharmacists; 2016:2013-2020.
7. Sweetman SC, ed. Martindale: The Complete Drug Reference. 35th ed. London, England: Pharmaceutical Press; 2007:2142.
8. Driver S. Propylene glycol. In: Sheskey PJ, Cook WG, Cable CG, eds. Handbook of Pharmaceutical Excipients. 8th ed. London, England: Pharmaceutical Press; 2017:795-798.
9. PCCA. PracaSil-Plus. www.pccarx.com/Products/ProductCatalog?search=pracasil. Accessed September 9, 2019.
To comment on this article, contact rdavidson@uspharmacist.com.





