Colorectal (colon) cancer is extremely common. Of all the cancers that affect both men and women, colorectal cancer is the second-leading cancer killer in the United States.1 The disease manifests in the colon or rectum, with 70% of colorectal cancer occurring specifically in the sigmoid colon and rectum.2,3 While colorectal cancer affects men and women of all racial and ethnic groups, cancer of the colon is more common in women, whereas cancer of the rectum is more common in men.2,3 The disease is most often found in adults aged 50 years or older, with incidence beginning to rise at age 40 and peaking at age 60 to 75.3 According to United States Cancer Statistics, in 2005 there were 141,405 people diagnosed with colorectal cancer in the U.S., and 53,005 deaths were attributed to it.1,4 Remarkably, up to 60% of deaths from this condition could be prevented.2 Routine screening, beginning at age 50, is the key to preventing colorectal cancer.5 Screening saves lives (TABLE 1).3
Clinical Features and Metastasis
Symptoms of colorectal cancer develop insidiously and often are present for months, even years, before diagnosis.6 Symptoms include blood in the stool or a change in bowel habits.3 Colorectal tumors spread into adjacent structures (i.e., by direct extension) and metastasize through the lymphatics and blood vessels.6 Sites of metastatic spread are, preferentially, the regional lymph nodes and the liver, lungs, and bones; many other sites may follow.6 Carcinomas of the anal region are locally invasive and involve metastasis to regional lymph nodes and distant sites.6
Staging, Prognosis, and Treatment
The extent of the tumor at time of diagnosis is the most important prognostic indicator of colorectal cancer.6 Staging designates the extent of the disease regarding tumor penetration, regional lymph node metastasis, and distant metastasis.7
Treatment for colorectal cancer is by surgical resection and chemotherapy (for lymph node involvement).3 Typical chemotherapeutic agents utilized are 5-fluorouracil and leucovorin; survival is improved by 10% to 30% when used in patients with colorectal cancer with positive lymph nodes.3 Combined radiation and chemotherapy may be beneficial in patients with rectal cancer with one to four positive lymph nodes.3 For improving the rate of resectability in rectal cancer or reducing lymph node metastasis, preoperative radiation and chemotherapy may be employed.3
Colorectal Cancer Screening Saves Lives
The premise for the utility of cancer screening, in general, is that early diagnosis may reduce cancer mortality, result in less radical therapy, and decrease costs.3 Colorectal cancer screening, in particular, is capable of detecting precancerous polyps in the colon or rectum for removal and can detect early-stage cancer so that treatment may be initiated when it is more effective, often leading to a cure.4,8 It is predicted that if all individuals aged 50 or older had regular colorectal screening tests resulting in the removal of all precancerous polyps, up to 90% of deaths from colorectal cancer could be prevented.4
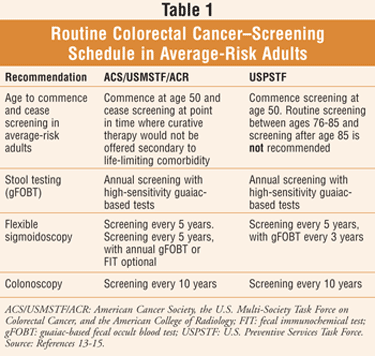
Screening for colorectal cancer begins soon after an individual turns 50 years of age, then continues at regular intervals (TABLE 1).8,9 People at higher risk for colorectal cancer should be tested at a younger age and/or more frequently, including individuals who 1) have a personal or close family history of colorectal polyps or colorectal cancer; 2) have inflammatory bowel disease; 3) have genetic syndromes such as familial adenomatous polyposis (FAP) or hereditary nonpolyposis colorectal cancer.8,10 Patients should be encouraged to speak to their health care provider to ascertain when they should begin screening and how often they should be tested.8
Screening Tests and Guidelines
There are several screening tests that can be used to detect polyps or colorectal cancer; while each can be used alone, they are sometimes employed in combination.11 The U.S. Preventive Services Task Force (USPSTF) recommends colorectal cancer screening in average-risk men and women aged 50 to 75 years using high-sensitivity fecal occult blood testing, sigmoidoscopy, or colonoscopy (TABLE 1).11
A high-sensitivity fecal occult blood test (FOBT) checks for occult blood in three consecutive stool samples.10,12 There are two types of FOBT: 1) guaiac based and 2) fecal immunochemical using antibodies. A test kit for home use can be provided by a health care practitioner. The patient employs a stick or brush to obtain a small amount of stool to be checked for an abnormality by the doctor or a laboratory.11 Routine schedule: annually.10,12
A flexible sigmoidoscopy is a procedure in which a physician uses a short, thin, flexible, lighted tube (sigmoidoscope) to visually inspect the interior walls of the rectum and only a portion of the colon; Routine schedule: every 5 years.10,12
A colonoscopy involves a flexible, lighted tube (colonoscope) with which the physician visually inspects the interior walls of the rectum and the entire colon; samples of tissue may be collected during the test for closer examination, and polyps may be removed as well. The colonoscopy is also used as a follow-up test if any previous result from one of the other screening tests is unusual.10-12 Routine schedule: every 10 years.10,12
Other screening tests not recommended by the USPSTF may be used in some settings, may be recommended by other groups, or are being studied.11 Health insurance plans usually do not cover these tests; additionally, if any unusual findings are noted, a follow-up colonoscopy is required.11 Examples include a double-contrast barium enema, consisting of a liquid barium enema followed by an air enema, which allows for a visual outline of the colon on an x-ray; a virtual colonoscopy, creating images of the entire colon on a computer screen through the use of x-rays and computer technology (while no sedation is necessary, bowel preparation is still required; lesions cannot be biopsied during this diagnostic procedure as in the optical colonoscopy); and a stool DNA test, requiring the collection of an entire bowel movement to be sent to a laboratory to test for cancer cells.7,11
A consensus guideline for colorectal cancer screening was released in March 2008 by the American Cancer Society, the U.S. Multi-Society Task Force on Colorectal Cancer, and the American College of Radiology (ACS/USMSTF/ACR), while the USPSTF updated its screening recommendations in October 2008.13-15 A concise and summarized version of the most current recommendations is available in TABLE 1; for complete and detailed coverage of the recommendations, refer to Reference 13 online. Ongoing studies drive the constantly evolving recommended screening schedules.3 The involved organizations promote a message for adults: Choose a test and get screened if you are 50 or older.13
The CDC indicates that the decision to be screened after age 75 should be made on an individual basis; patients older than 75 are instructed to consult their physician as to whether they should be screened.11 The USPSTF suggests that routine screening between ages 76 and 85 years is not recommended; screening after age 85 is also not recommended.13 According to the ACS/USMSTF/ACR consensus guideline for colorectal cancer screening, screening should end at a point where curative therapy would not be offered due to life-limiting comorbidity.13
Demonstration Project
Partnerships have been built by the CDC to encourage screening, support education and training, and conduct surveillance and research.4 One particular program developed by the CDC is the Colorectal Cancer Control Program, which provides funding to 26 states and tribes across the U.S.4 It provides colorectal cancer–screening services to low-income men and women aged 50 to 64 years who do not have insurance or are underinsured for screening when no other insurance options are available. States and tribes in the CDC’s Colorectal Cancer Control Program can be found in the map provided in
Reference 4 online.
Conclusion
Symptoms of colorectal cancer develop insidiously and often are present for months, and maybe years, before they are diagnosed. While screening for colorectal cancer saves lives, the disease remains extremely common. Pharmacists, in the name of health promotion, disease prevention, and patient advocacy, should encourage individuals who are aged 50 or older or who think they may be at higher-than-average risk for colorectal cancer to speak to their doctor about getting screened. Routine screening is the key to preventing colorectal cancer.
REFERENCES
1. Centers for Disease Control and Prevention. Colorectal (colon) cancer. www.cdc.gov/cancer/Colorectal. Accessed November 16, 2009.
2. Centers for Disease Control and Prevention.
Basic information about colorectal (colon) cancer.
www.cdc.gov/cancer/colorectal/basic_info/index.htm. Accessed November 16, 2009.
3. Beers MH, Porter RS, Jones TV, et al. The Merck
Manual of Diagnosis and Therapy. 18th ed. Whitehouse Station, NJ: Merck Research Laboratories; 2006:89-94,173-178,1147-1150.
4. Centers for Disease Control and Prevention. Colorectal Cancer Control Program. www.cdc.gov/cancer/crccp. Accessed November 16, 2009.
5. U.S. Preventive Services Task Force. Screening for Colorectal Cancer: U.S. Preventive Services Task Force Recommendation Statement. Rockville, MD: Agency for Healthcare Research and Quality; October 2008. AHRQ publication 08-05124-EF-3.
6. Cotran RS, Kumar V, Collins T. Robbins Pathologic Basis of Disease. 6th ed. Philadelphia, PA: WB Saunders Company; 1999:834-835.
7. The Merck Manuals Online Library. Colorectal cancer. Table 2. Staging colorectal cancer. Revised December 2007. www.merck.com/mmpe/sec02/ch021/ch021h.html. Accessed November 17, 2009.
8. Centers for Disease Control and Prevention. Colorectal cancer screening. www.cdc.gov/cancer/colorectal/basic_info/screening. Accessed November 16, 2009.
9. Comparison of 2008 ACS/USMSTF/ACR guidelines with those of the USPSTF. www.cancer.org/docroot/PRO/content/PRO_4_1x_ColonMD_Comparison_Guidelines.asp. Accessed November 16, 2009.
10. Centers for Disease Control and Prevention. Colorectal cancer screening guidelines. www.cdc.gov/cancer/colorectal/basic_info/screening/guidelines.htm. Accessed November 16, 2009.
11. Centers for Disease Control and Prevention. Colorectal cancer screening tests. www.cdc.gov/cancer/colorectal/basic_info/screening/tests.htm. Accessed November 16, 2009.
12. U.S. Preventive Services Task Force. Guide to Clinical Preventive Services: Recommendations of the U.S. Preventive Services Task Force. Rockville, MD: Agency for Healthcare Research and Quality; September 2008. AHRQ publication 08-05122.
13. Comparison of 2008 ACS/USMSTF/ACR guidelines with those of the USPSTF. www.cancer.org/docroot/PRO/content/PRO_4_1x_ColonMD_Comparison_Guidelines.asp. Accessed November 16, 2009.
14. Levin B, Lieberman D, McFarland B, et al. Screening and surveillance for the early detection of colorectal cancer and adenomatous polyps, 2008: a joint guideline from the American Cancer Society, the US Multi-Society Task Force on Colorectal Cancer, and the American College of Radiology. CA Cancer J Clin. 2008;58:130-160.
15. U.S. Preventive Services Task Force. Screening for colorectal cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2008;149:627-637.
To comment on this article, contact rdavidson@jobson.com.





