US Pharm. 2024;49(6):28-34.
ABSTRACT: Biosimilars have been gaining traction as a cost-effective alternative to biological products. Patients, physicians, and pharmacists have differing perceptions of what biosimilars are and their evolving roles in therapy choice. Insurance-related barriers are also present and may pose a challenge to access for patients who are prescribed biosimilars. Biosimilar uptake, in general, has been inconsistent, and part of this is believed to be the result of a gap in healthcare provider and patient understanding and the biosimilars’ emerging roles in treatment. Pharmacists can provide education to patients, physicians, and other pharmacists about biosimilars and help navigate some of these obstacles to improve patient care. Pharmacist self-assessment of biosimilar knowledge has also been studied, and some points of improvement for this have been identified.
Increasingly, biosimilars are a cost-effective option for patients who are taking biologic medications. Per the FDA, biosimilars are defined as “a biologic medication that is highly similar to and has no clinically meaningful differences from an existing FDA-approved biologic, called a reference product.” Compared with a reference product, biosimilars are 1) made with the same types of living sources, 2) are given to the patient in the same way, and 3) have the same strength, dosage, potential treatment benefits, and potential side effects.1 Although similar to generic medications in some ways, the active ingredients of biosimilars are more difficult to copy due to protein variability. Biosimilars are very cost-effective compared with their parent products, as they do not have to be reevaluated from the ground up for efficacy.
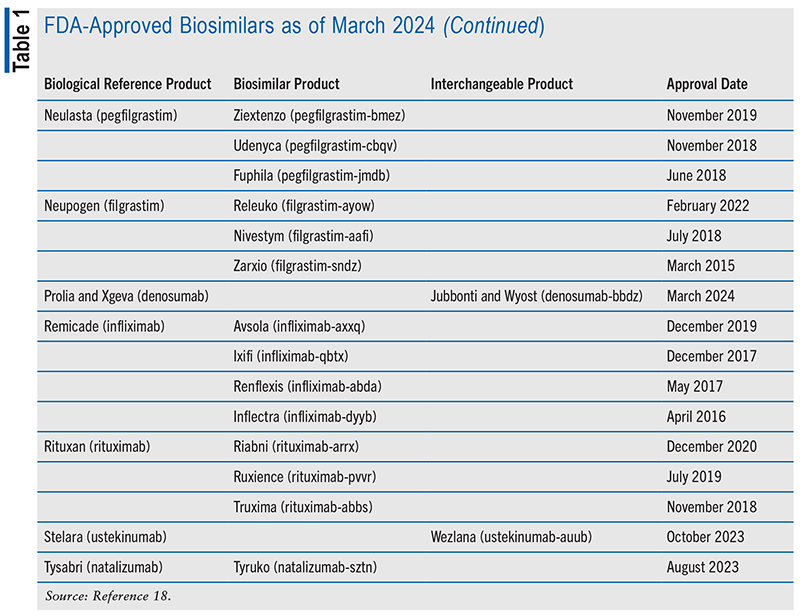
As part of the FDA’s regulatory process, biosimilars are evaluated alongside the parent product for biosimilarity to demonstrate efficacy and safety. Human pharmacodynamic and pharmacokinetic trials are specified by the FDA as “fundamental components” when submitting an application for a potential biological product. Manufacturers can undergo additional testing and apply for an “interchangeable biosimilar,” which—depending on various state laws—pharmacists can dispense to patients as they would a generic versus brand-name substitution (see TABLE 1 for examples of biological agents and their respective biosimilar and interchangeable products). Therefore, pharmacists may find themselves in the position of counseling patients about the differences between these options as well as any pharmacy insurance-related information that may initiate the switch. A primary driving factor for this substitution is the reduced costs associated with acquiring biosimilars, resulting in larger prescription drug savings. Biosimilars are estimated to have saved $23.6 billion in prescription drug spending since the first FDA-approved biosimilar launched in 2015, and a significant portion of this total ($9.4 billion) was saved in 2022 alone.2 This has been primarily accomplished by biosimilar-driven market competition, often resulting in reduced prices for the originating biologic medications.2 Biosimilars are estimated to save at least $38.4 billion in healthcare expenditures between 2021 and 2025.3 Further, algorithms have predicted possible savings of up to $124.2 billion under potential scenarios of biosimilars driving down reference product prices and being available at lower costs compared with when they are initially released into the market.3 The biosimilar impact of driving down original biologic prices was noted to be the most impactful factor in the savings predictions, which is consistent with their influence on the biologic medicine pricing landscape up to this point.

Patient and Physician Perceptions of Biosimilars
A systematic review of physicians’ perception of biosimilars was conducted in multiple parts of the world with access to biosimilars; the majority of the included studies were based in Europe and the United States.4 The authors aimed to assess different physician perceptions of biosimilars and how this influenced his or her utility (or lack of) in daily medical practice. Physicians who had generally positive opinions of biosimilars cited several reasons that would influence their decision to prescribe a biosimilar. Some reasons included a regulatory body, such as the FDA or European Medicines Agency, holding biosimilars to the same standards as the original product in regard to safety and efficacy, high-quality postmarket pharmacovigilance studies, and increased access and lower cost to patient.4 Physician specialty also influenced their willingness and comfortableness to prescribe biosimilars, with European gastroenterologists, dermatologists, endocrinologists, and rheumatologists stating more willingness either to switch a patient to a biosimilar or to initiate one for treatment over the originator’s product.4
Physicians who voiced negative opinions or were mistrustful of biosimilars raised concerns over safety and efficacy, immunogenicity, and lack of clinical data. Baseline knowledge of biosimilars varied greatly between studies and was thought to be a reason for their reduced utilization in practice. Academic detailing, or direct education to prescribers provided by another healthcare professional (e.g., a pharmacist), has demonstrated increased prescribing of other medications (e.g., antibiotics).5 Additionally, many physicians reported hesitation about and disagreed with pharmacist-led substitution of biosimilars for the originator products since the physicians did not view them as comparable with generic drugs. If more evidence for pharmacist-led substitution was provided, prescribers stated that they would consider this practice, and many believed that in time it will be allowed in the U.S.—similar to generic drug substitution.4 Therefore, current knowledge of biosimilars is necessary for pharmacists to help prescribers navigate substitution of these products when it is required or preferred by the patient.
Patients have also been surveyed regarding their perceptions of biosimilars. Patients who had been initiated on a reference product were generally open to switching to a biosimilar depending on certain circumstances.6 For example, patients with psoriasis or rheumatoid arthritis were more likely to switch to a biosimilar of etanercept when offered. The patients who were more likely to refuse the switch were older, had a longer disease course, or received negative information from either his or her pharmacist or physician about the biosimilar.6 Across multiple studies, patients were reported to be more unlikely to switch medications if the primary reason was for a nonmedical switch. If out-of-pocket cost was higher, patients were more likely to switch for any reason, including nonmedical switches.
Patients who were unwilling to switch across multiple studies cited that the main reason for refusal was that he or she believed the original products were superior in terms of quality, efficacy, and safety, or they felt there were not enough clinical data or pharmacovigilance reports for biosimilars compared with their reference products. Some patients were also concerned with the possibility of or had directly experienced symptom exacerbation or side effects after switching and were unwilling to accept a second substitution.7 Other patients were satisfied with their original biologic prescriptions in regard to symptom control and did not want to switch to a biosimilar for a nonmedical reason. Some studies have shown a correlation between lower medication prices and patients believing that this decreased cost was due to inferiority to the original.7 Generally, patient self-assessment of biosimilar knowledge was poor or lacking, and there seems to be additional room for education.6
Certain factors were found to influence an increased patient baseline knowledge of biosimilars. These included younger age, previous treatment with either a parent biologic or biosimilar, and active membership in patient organizations. One study stated that in many instances, patients felt underinformed by their prescribers. There were circumstances in which patients were given an introduction and instructions for the initiation of the parent adalimumab product but were not informed of the biosimilar product when switched or instructed on how to use it.8 Although both medication dosage forms had the same route of administration, the differences between the dosage forms (e.g., needle size) were patient-reported as negative experiences that could have been alleviated by proper counseling. The sources of information patients use can also influence their perceptions and utilization of biosimilars. Physicians, pharmacists, and academic sources were listed as among the most reputable sources of information, and information found on the Internet by patients was associated with greater concerns and reluctance to switch biologic medications.9
The Pharmacist’s Role in Biosimilar Knowledge
As medication experts, pharmacists are primed to educate both providers and patients about biosimilars. Counseling points of interest to patients should include the name of the biosimilar in question, the FDA-approved indication, and the similarities to the reference product.10 These similarities include the benefits of the medication, the identical strength and dosage form, and expected adverse-effect profile, which should not differ meaningfully between the reference product and its biosimilar. Differentiating drug names can be helpful for both providers and patients, as unlike generic drugs, biosimilars have their own trade names. Historically, generic medications were not widely accepted upon their first appearance in the 1960s, but due to consumer demand for more affordable products, their use became more widespread.
There is an emerging role for clinical pharmacists in the uptake and subsequent education needed for biosimilars to reach a higher utility rate, as many of the medications are prescribed by clinical specialists.10 Clinical pharmacists practicing in a hospital, for example, may prompt initiatives to increase biosimilar use across hospital formularies. Patients receiving these medications while hospitalized or when presenting to outpatient clinics would subsequently have greater access to these therapies, as well as pharmacists to assist the patient’s healthcare team in making these treatment decisions. Pharmacists in a hospital setting may be called upon to provide counseling and education by physicians, nurses, other pharmacists, pharmacy technicians, pharmacy interns, and patients.10
Surveys have been conducted assessing a pharmacist’s perceptions of his or her own knowledge of biosimilars. One survey taken in 2021 collected data from 500 pharmacists across the U.S. to assess their understanding of biosimilars.11 This included their knowledge of the FDA’s approval process for biosimilars, how they are regulated, and when they can be substituted as interchangeable products. Naming of the biosimilars and patient involvement in decision-making were also assessed. Many of the pharmacists in the survey worked in a community pharmacy setting, two-thirds had been licensed and were working as pharmacists for more than 10 years, and their practice settings were fairly evenly distributed across the Northeast, Midwest, South, and West regions of the U.S. A high percentage of the pharmacists were confident that biosimilars would perform similarly to their reference products and that they were not necessarily riskier to use or less effective.11 Many pharmacists (80%) were not aware of the ability to substitute an interchangeable biosimilar without first contacting the prescriber of the medication.11 More hospital or health-system pharmacists than those working in a community setting were not aware that the FDA approval of a biosimilar does not automatically classify it as an interchangeable product. This is possibly because hospital formularies generally set policies for equivalence within the hospital that may not equate to an automatic switch in circumstances outside of the facility.11 At the time that this study was conducted, there were no FDA-approved interchangeable biosimilars; there are currently 10 that are approved (see TABLE 1). Over 90% of pharmacists in the survey understood the biosimilar laws most similar to the ones for generic drugs and substitution, such as the prescriber requesting only the brand-name medication (or in this case, the original biological therapy).11 Additionally, only about 50% of the pharmacists surveyed felt “very or moderately comfortable” answering patients’ biosimilar questions, and this identifies an area of need.11 Knowledge gaps about the clinical and regulatory processes of interchangeability and substitution may close over time as more interchangeability studies comparing biologics directly alongside biosimilars become available.
Insurance-Related Barriers and Counseling Points
Insurance often prompts providers to discuss treatment options with patients. Given the relatively recent emergence of biosimilars in the U.S., navigating health insurance hurdles can be challenging at times for patients and providers. Patterns in barriers related to insurance have been identified in the existing literature. One study explored reasons for insurance exclusion among all of the available biosimilar products that the FDA approved up to 2021.12 One of the primary reasons patients may have a biosimilar substituted for their pharmacotherapy is the lower cost of treatment, which will ideally allow a wider cohort of patients access to this biological therapy. Data from 17 of the 20 largest private insurers in the U.S. were collected for this analysis. The authors found that 19.4% of the coverage decisions collected imposed step-therapy or plan exclusions.12 Biosimilars indicated for cancer treatment were less likely to be restricted across plans. Biologic medications intended for use in the pediatric population were more likely to have restrictions imposed on biosimilars compared with reference products. On average, the first biosimilar to reach the market generally encounters the least restrictive plan coverage and has more time to establish long-term safety data compared with competitor products that will inevitably follow initial biosimilar FDA approval. Cost savings of $15,000 or greater per patient were identified to be another factor in favor of insurance coverage, especially if cost-effectiveness data were provided for a biosimilar product.13 If the reference value of the original product is not readily available, plans are much more likely to cover the biosimilars. Biosimilars that are FDA-approved to treat diseases with higher prevalence (e.g., inflammatory conditions) were another commonly observed reason for insurance rejection. Although biosimilar use for highly prevalent illnesses has great potential for increased cost savings and increased patient access, insurance plans may have already negotiated more favorable prices with manufacturers of the original reference biologic medications. These diseases are likely to result in higher amounts of insurance plan spending for medical care, and the decreased cost of reference biologics provides plan incentive to not switch to other options if the cost savings are not substantially different.14 Finally, if insurance coverage of the reference product is very restrictive, this barrier is likely to pass on to any future biosimilars indicated to treat the same disease. This study focused on privatized health plans and is not reflective of any government-funded insurance plans, such as Medicare, Medicaid, or Veterans Affairs.
For medications dispensed from pharmacies, pharmacists should be prepared to counsel patients on various insurance-related barriers and how to navigate them. Pharmacists working for healthcare plans should also be prepared to source the appropriate data and calculate expected saved costs. Tools like BIOSIM.CARE can be utilized to determine insurance coverage and identify potential insurance-related barriers prior to the patient presenting to the pharmacy.15 Knowing this information ahead of time can accelerate access to these medications.
Biosimilars have been in use in Europe since 2006, starting with the European Union’s approval of Omnitrope (somatropin). A comprehensive literature review aimed to define the responsibilities and outlines five strategies utilized to counsel patients about biosimilars: 1) provide understandable information, 2) in a positive and transparent way, 3) tailored to the individual’s needs, 4) with one voice, and 5) supported by audiovisual material.16 The authors warn of the nocebo effect, or “the worsening of symptoms associated or an increase in side effects with a negative attitude towards a given therapy,” that may occur when a biosimilar is substituted for the original product that a patient was taking for a treatment. Patient misunderstandings about what biosimilars actually are can worsen this phenomenon. Although it is still unclear what information is necessary to include for patient education about biosimilars, healthcare providers should be prepared to provide counterpoints to misinformation patients can often encounter while reading online.17 Individual patient concerns should also be addressed as they come up in conversation, as some patients may be worried more about side effects while others may believe that they are getting an inferior product in regard to efficacy. Regardless of individual patient concerns, comparable safety and efficacy should be highlighted. If cost savings are the only benefit mentioned, patients may believe that they are getting a lower-quality product when strict safety and efficacy standards for marketing are in place for biosimilars. Similar to other medications, biological reference products have patents that expire, which allows for market competition that drives down drug costs and prices. All healthcare providers involved in the patient’s care should be able to provide consistent information about the biosimilar he or she is prescribed. Pharmacists were identified as being a potential first point of contact for patients taking biologic medicines, as insurance-related changes can prompt a discussion about biosimilars. Pharmacists are able to increase patient confidence in these medications as well as teach patients how to self-administer injectable dosage forms.
Pharmacists should be prepared to counsel patients about biosimilar interchangeable products, as well as their approval pathway and postmarketing monitoring. Patients should be counseled about adverse effects of biosimilars as well as their cost-effectiveness and inevitable wider use across healthcare. Although they are relatively new to the U.S., they have been in use for longer periods of time across Europe, and there is an existing body of safety data from studies conducted there. These studies indicate that patients are more likely to accept biosimilars when they are more informed about them. This can consist of the individual’s understanding of the regulatory and approval process as well as pharmacovigilance or postmarketing information on how safe and effective the drugs are. In addition to patient support and education, pharmacists should be prepared to share information with physicians and, depending on individual state laws, discuss interchangeable biosimilars with the physician so that her or she is aware that patients are dispensed the correct medication. As more interchangeable biosimilars are FDA-approved, their uptake is likely to increase, as pharmacists will have more authority to dispense the medications without excessive calls to the prescriber. While state license requirements can vary, pharmacists should consider completing some biosimilar-focused continuing education credits to expand their knowledge base of this evolving field. Additionally, patients may ask questions about biosimilar efficacy, cost, differences between the original biologics, adverse effects, and proper administration, as these scenarios can differ depending on the preparation prescribed.
Conclusion
Pharmacists can counsel patients, physicians, and even other pharmacists about biosimilar interchangeability- and insurance-related barriers. More studies are likely needed as policies around biological drugs continue to evolve. Literature suggests that physicians and pharmacists alike feel more comfortable with biosimilars the more familiar they are with the clinical and regulatory process. Additionally, insurance-related barriers can often present an obstacle to patient access. Some observed patterns in insurance restrictions have included biosimilars used in the pediatric population, biosimilars that are newer to the market, biosimilars indicated for highly prevalent diseases, and biosimilars that have very restricted parent biologics. Conversely, there are biosimilars indicated for cancer treatment, biosimilars that produced savings of $15,000 or greater, biosimilars that have been on the market for longer periods of time, and biosimilars that had cost-effectiveness data that were noted to be covered more often by insurance plans. When counseling patients on biosimilars, pharmacists should provide understandable information in a positive and transparent way that is tailored to the individual’s needs in collaboration with the patient’s prescriber and, when possible, supported with audiovisual material and any educational material that may assist all involved to make the most informed decision possible.1
REFERENCES
1. FDA. Biosimilars: overview for health care professionals. January 18, 2024. www.fda.gov/drugs/biosimilars/overview-health-care-professionals. Accessed May 2, 2024.
2. Association for Accessible Medicines. The U.S. Generic & Biosimilar Medicines Savings Report, September 2023. https://accessiblemeds.org/sites/default/files/2023-09/AAM-2023-Generic-Biosimilar-Medicines-Savings-Report-web.pdf. Accessed May 2, 2024.
3. Mulcahy A, Buttorff C, Finegold K, et al. Projected US savings from biosimilars, 2021–2025. Am J Manag Care. 2022;28(7):329-335.
4. Sarnola K, Merikoski M, Jyrkkä J, Hämeen-Anttila K. Physicians’ perceptions of the uptake of biosimilars: a systematic review. BMJ Open. 2010;10:e034183.
5. Solomon DH, Van Houten L, Glynn RJ, et al. Academic detailing to improve use of broad-spectrum antibiotics at an academic medical center. Arch Intern Med. 2001;161:1897-1902.
6. Wu Q, Wang Z, Wang X, et al. Patients’ perceptions of biosimilars: a systematic review. BioDrugs. 2023;37:829-841.
7. Scherlinger M, Langlois E, Germain V, Schaeverbeke T. Acceptance rate and sociological factors involved in the switch from originator to biosimilar etanercept (SB4). Semin Arthritis Rheum. 2019;48(5):927-932.
8. Karlsdottir K, Gunnarsdottir AI, Grondal G, et al. A patients’ perspective towards the injection devices for Humira® and Imraldi® in a nationwide switching program. Front Med (Lausanne). 2022;9:799494.
9. Vandenplas Y, Barbier L, Simoens S, et al. Perceptions about biosimilar medicines among Belgian patients in the ambulatory care. Front Pharmacol. 2022;12:789640.
10. Okoro RN. Biosimilar medicines uptake: the role of the clinical pharmacist. Explor Res Clin Soc Pharm. 2021;1:100008.
11. Stevenson JG, McCabe D, McGrath M, McBride A. Pharmacist biosimilar survey reveals knowledge gaps. J Am Pharm Assoc (2003). 2023;63(2):529-537.e7.
12. Yu T, Jin S, Li C, et al. Factors associated with biosimilar exclusions and step therapy restrictions among US commercial health plans. BioDrugs. 2023;37(4):531-540.
13. Mestre-Ferrandiz J, Towse A, Berdud M. Biosimilars: how can payers get long-term savings? Pharmacoeconomics. 2016;34:609-616.
14. Hakim A, Ross JS. Obstacles to the adoption of biosimilars for chronic diseases. JAMA. 2017;317:2163-2164.
15. Chambers JD, Lai RC, Margaretos NM, et al. Coverage for biosimilars vs reference products among US commercial health plans. JAMA. 2020;323(19):1972-1973.
16. Vandenplas Y, Simoens S, Van Wilder P, et al. Informing patients about biosimilar medicines: the role of European patient associations. Pharmaceuticals (Basel). 2021;14(2):117.
17. Tan SSL, Goonawardene N. Internet health information seeking and the patient-physician relationship: a systematic review. J Med Internet Res. 2017;19(1):e9.
18. FDA. Purple Book database of licensed biological products. https://purplebooksearch.fda.gov/.
The content contained in this article is for informational purposes only. The content is not intended to be a substitute for professional advice. Reliance on any information provided in this article is solely at your own risk.
To comment on this article, contact rdavidson@uspharmacist.com.






