US Pharm. 2014;39(2):36-39.
ABSTRACT: When pharmacologic treatments fail to treat major depression, electroconvulsive therapy (ECT) becomes a powerful and effective alternative. ECT involves using anesthesia plus neuromuscular blocking agents to alleviate its adverse effects. The two agents most commonly used in ECT anesthesia are methohexital and propofol. The ideal anesthetic agent should have a rapid onset of action and short recovery time. Further, it should be able to maintain electroencephalographic (EEG) seizure activity that is adequate to elicit optimal antidepressant effects. The pharmacokinetic properties of the anesthetic agent determine the duration of therapy. The main problem with these agents is their anticonvulsive properties; therefore, the effects on seizure duration, strength of the stimulus charge, and recovery times after each treatment are important. All of these factors must be taken into consideration while managing the patient’s long-term cognitive complications.
Electroconvulsive therapy (ECT) is considered an acceptable treatment for major depressive disorder, especially when it has become resistant to pharmacotherapeutic and nonpharmacotherapeutic treatments.1 Over the years, ECT has been considered an unorthodox form of treatment for these patients. However, it is rapidly effective and can be a life-saving treatment for patients with suicidal ideation.
ECT is an effective form of treatment with acceptable side effects when performed with the appropriate anesthetics. General anesthesia management during a surgical procedure differs from anesthesia management during ECT due to a shorter duration and higher complexity of treatment. Many of the available anesthetic agents such as barbiturates and benzodiazepines possess anticonvulsant properties that affect seizure duration and threshold. These agents directly affect clinical outcomes in ECT treatments.
Determining the ideal anesthetic agent is crucial during ECT for the patient’s quality of life. General anesthesia is required to perform ECT and is induced with agents such as methohexital, propofol, thiopental, etomidate, ketamine, benzodiazepines, and sevoflurane. These agents are used in conjunction with neuromuscular blocking agents (NMBs) such as succinylcholine, mivacurium, atracurium, rocuronium, and rapacuronium.2 Succinylcholine is the most commonly used NMB to dampen the muscle contractions that are induced by ECT.3 To date, deciding the optimal induction agent has been difficult because of the widespread acceptance of methohexital as the gold standard of therapy.3
Background
The exact mechanism of action of ECT in the treatment of resistant psychiatric disorders remains unclear. ECT induces a generalized, tonic-clonic epileptic seizure that is used to treat severe, resistant depression.2 In 1938, the first ECT procedure was performed without the use of an anesthetic.3 The idea of applying a dangerously high current of electricity is still associated with ECT, but nowadays the treatment is safe and consistent when received under anesthesia. The electrical current during ECT is administered in a controlled setting with staff trained to monitor for certain adverse effects such as changes in blood pressure and heart rate. Currently, general anesthesia is used with the goal of quick onset and offset of unconsciousness and of muscle relaxation for the duration of the electrical stimulation and seizure. The overall objective of the anesthetic agent is to make the patient unconscious, which allows the patient to be unaware of the sensations during therapy. This is done while trying to minimize physiological and physical effects of the procedure.
The duration of an ECT-induced seizure is dose-dependent. The anesthetic agents with higher anticonvulsant characteristics will require a larger dose, which would reduce the duration of the seizure.3 The anesthetics of choice have anticonvulsant characteristics, making it a fine balancing act between suitable anesthetic coverage and negative effects on therapy.
During ECT treatment, an electrical current is applied transcutaneously to the brain via two electrodes.2 These electrodes can be positioned bilaterally or unilaterally.2 Bilateral ECT involves electrodes placed bitemporally, while unilateral ECT has two electrodes placed temporoparietally.2 Maximizing the distance between the electrodes reduces shunting of the electrical current.2 Bilateral ECT is the preferred position due to speed of recovery, but unilateral ECT has lower cognitive adverse effects such as disorientation, impaired attention, and memory problems because the electrodes are placed on the nondominant hemisphere.2 This results in a generalized motor seizure with an electroencephalographic (EEG) spike that leads to an increase in cerebral blood flow and intracranial pressure.3 The generalized seizure duration has not been established, but a short seizure (<10 seconds) or a very long seizure (>120 seconds) could be associated with lower clinical efficacy.2 Some research suggests that it is not the length of the seizure that is important but the strength of the electrical current being used.
ECT is recommended twice weekly until lack of improvement, which can average around 3 to 4 weeks.2 ECT can cause a parasympathetic-induced bradycardia, followed by tachycardia and hypertension lasting several minutes. In addition, the response increases systolic blood pressure and heart rate, which results in increased myocardial oxygen depletion.3
A common adverse effect following ECT is memory loss. Other serious adverse effects are myocardial ischemia, infarctions, transient neurologic ischemic deficits, intracranial hemorrhages, and cortical blindness.3 The ECT-induced seizures have been known to also cause fractures and muscle aches due to convulsions from therapy.3 Other problems such as emergence agitation, nausea, headache, and even death have also occurred after ECT.3
The goal of ECT is to produce an EEG seizure that lasts long enough to elicit an optimal antidepressant effect.2 Seizures can be induced pharmacologically, but effects are not consistent, and it can also induce a sense of panic in the patient.4 By controlling when the seizure will occur, patients are better able to plan their schedules. Not only is it convenient for the patient, but ECT has been found to be more efficacious than pharmacologic therapy at inducing seizures and decreasing the number of missed or recurrent seizures.4
Anesthetic Agents
According to the American Psychiatric Association, methohexital is the anesthetic of choice because it has an established safety record, is effective, and has low costs.5 In 2002, there was a nationwide recall of methohexital, rendering delays in manufacturing and forcing a switch to alternative products, including propofol, thiopental, etomidate, ketamine, benzodiazepines, and sevoflurane.6 When thiopental was compared with methohexital, it showed a frequency of increased sinus bradycardia and premature ventricular contractions.3 Etomidate is associated with longer seizure duration, delayed recovery from post-ECT confusion, and higher nausea and vomiting symptoms when compared to methohexital and propofol.3 Ketamine is an anesthetic agent with analgesic properties that are less desirable due to its ability to increase intracranial pressure.3 Benzodiazepines should be ruled out as an option because of their noticeable anticonvulsant activity.3 Sevoflurane is a volatile anesthetic agent with similar anesthetic characteristics to thiopental, but there is not an advantage to using this agent because it is more time consuming to administer.3 Out of the listed agents, methohexital and propofol have become the most widely used anesthetic agents when available (TABLE 1).
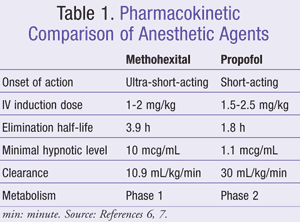
Methohexital: Methohexital is an ultra-short-acting barbiturate and is considered the drug of choice in ECT anesthesia.6 This medication is rapidly cleared and accumulates less than most of the barbiturates during extended infusions.7 The elimination half-life of methohexital is 3.9 hours at an IV induction dose of 1 to 2 mg/kg.7 The minimal hypnotic level is 10 mcg/mL, and medication clearance is 10.9 mL/kg/min.7 Methohexital is eliminated by phase 1 hepatic metabolism followed by renal excretion of inactive metabolites.7 What makes methohexital an ideal anesthetic agent for ECT is that it increases ictal activity and can produce suppression of the EEG spike.7 There is a dose-dependent decrease in blood pressure with methohexital due to venodilation and injection site pain experienced during the treatment, which can be reduced with prior lidocaine injection.7
Propofol: Propofol is a short-acting hypnotic with a pharmacokinetic profile similar to thiopental’s, which makes it a good agent for recovery after anesthesia.6,7 The elimination half-life of propofol is 1.8 hours at an IV induction dose of 1.5 to 2.5 mg/kg.7 The minimum hypnotic level is 1.1 mcg/mL and the medication clearance is 30 mL/kg/min.7 Propofol has a very high clearance and short duration of action, which makes it a good anesthetic agent for rapid discharge after treatment.7 The agent is cleared by phase 2 hepatic conjugation to sulfate and glucuronide, which are then renally cleared.7 Due to propofol’s high plasma protein binding, conditions such as cirrhosis will increase its elimination time.7 Propofol produces its anesthetic effects by acting on gamma-aminobutyric acid type A (GABAA) receptors to hyperpolarize neurons, leading to suppression of the EEG spike.7 A dose-dependent blood pressure drop is seen because of vasodilation. Just as with methohexital, there is pain on injection, which can also be mitigated with prior lidocaine administration.7
Discussion
Because of its established use, methohexital is recommended as the induction agent of choice for ECT.1,6 A retrospective study by Vaidya et al showed that when using right unilateral (RUL) probe placement during ECT anesthesia, there was a shorter ECT course, a lower average stimulus charge required, and a longer average seizure duration than with propofol.6 This anesthetic agent has become the preferred agent because of its pharmacokinetic profile. In addition, methohexital with all ECT courses had a lower average stimulus charge of 334 (140) mC than propofol 380 (122) mC (P = .02). Values are reported as mean (SD). The average seizure duration of ECT course with methohexital and propofol was 44.1 (17.9) seconds and 29.7 (8.5) seconds (P <.0001), respectively. There were also a higher number of restimulations when propofol was used. When RUL ECT courses were compared with all ECT courses, they displayed similar treatment characteristics. It is important to note that with RUL ECT courses, there was an average stimulus charge of 270 (129) mC (P = .03).6 With a lower average stimulus charge, there should be fewer negative effects from ECT therapy.
Of note, the Mini-Mental State Examination (MMSE) scores before and after ECT treatments for methohexital (-0.6 [3.0]) and propofol (0.0 [3.1]) were not statistically significant (P = .25). The Montgomery-Asberg Depression Rating Scale (MADRS) was not statistically significant between methohexital (-15.6 [12.9]) and propofol (-14.3 [9.1]) (P = .46).6
Propofol is also associated with increased risk of missed seizure when compared with methohexital. Although it was the anesthetic agent requiring the most energy, propofol also had a shorter seizure duration.4 When methohexital and propofol were compared at equal doses of 0.75, 1.0, and 1.5 mg/kg, they both produced dose-dependent reductions in motor and EEG seizure durations.1 A propofol dose of 0.75 mg/kg was associated with seizure durations similar to those of methohexital. The seizure duration of propofol is significantly shorter than methohexital’s when the dose is increased up to 1.5 mg/kg.3 Regardless of which drug was used, the awakening times were similar with both agents.1 There has been an association of improved cognitive performance after propofol anesthesia versus methohexital. However, there were only two cognition trials that were statistically significant in Geretsegger et al.8 The benefit seen with using propofol over methohexital is the quicker emergence from anesthesia. There is also a cardiovascular depressant effect seen with propofol as compared to methohexital, etomidate, and thiopental.3 With a greater average stimulus charge and proportion of failed seizures, propofol still resulted in a clinically acceptable seizure.3,9
It must be noted that propofol may be associated with a longer treatment course and higher patient costs.9 If seizure threshold were not a problem, propofol could be the preferred treatment for ECT anesthesia, but its anticonvulsant properties shorten seizures by increasing the seizure threshold.10
There was a nationwide shortage of methohexital in 2002 and 2003, and propofol was used instead at medical institutions nationwide.6 At Johns Hopkins Hospital in Baltimore, Maryland, for example, propofol was used until 2006, when patients were switched back to methohexital.6 The main concern was that propofol was raising the seizure threshold and increasing the number of times that restimulation was required for brief seizures. The methohexital shortage allowed for the chance to explore other agents that could possibly be used, thus allowing for propofol and the other drugs to be tested more extensively for ECT anesthesia. Methohexital is considered the “original and best” until propofol can be proven to not interfere with clinical efficacy. ECT anesthesia should be done with the agent of choice until more data are found to show it inferior to another agent.10
Deciding whether methohexital or propofol is the preferred induction agent has been difficult to conclude due to inconsistent and inconclusive studies. This circumstance may bring into question the use of other anesthetic agents. Etomidate could be used for patients with high seizure thresholds or short seizures leading to subtherapeutic effects.10 Ketamine has sympathomimetic activity that can make it less favorable versus the other agents.10 Benzodiazepines are usually used for their anticonvulsant and sedative properties, but for ECT anesthesia induction, they become less favorable.10 Sevoflurane can be advantageous if it is used to decrease post-ECT contractions in women who need ECT toward the end of their pregnancy.3,10
Conclusion
There are limited studies comparing methohexital and propofol for clinical efficacy. During drug shortages, providers have been forced to switch from methohexital to propofol or other agents. By comparing the pharmacokinetic profile of these drugs, however, it is clear that methohexital is an ideal anesthetic agent for ECT. The fact that methohexital does not increase the seizure threshold and decrease the seizure duration makes it a better choice than propofol for ECT anesthesia. Propofol should be regarded as a second-line anesthesia agent for ECT because of its potent anticonvulsant effects. If a generalized seizure can be induced with a lower current, it could increase the patient’s overall quality of life by decreasing the effects on cognitive function. When RUL ECT therapy is performed, the current is even lower than with bilateral ECT therapy; if this remains consistent, it could be the determining factor in choosing the ideal agent for anesthesia.
Methohexital is associated with fewer treatments for the mitigation of depression when compared to propofol. This could save patients time and money. As healthcare providers strive to provide their patients with the most efficacious treatments, it is very important to try to alleviate the side effects that are detrimental to the patient’s quality of life.
REFERENCES
1. Avramov MN, Husain MM, White PF. The comparative
effects of methohexital, propofol and etomidate for electroconvulsive
therapy. Anesth Analg. 1995;81:596-602.
2. Uppal V, Dourish J, Macfarlane A. Anaesthesia for electroconvulsive therapy. Contin Educ Anaesth Crit Care Pain. 2010;10:192-196.
3. Ding Z, White PF. Anesthesia for electroconvulsive therapy. Anesth Analg. 2002;94:1351-1364.
4. Mankad MV, Beyer JL, Weiner RD, Krystal A. Clinical Manual of Electroconvulsive Therapy. Arlington, VA: American Psychiatric Publishing, Inc; 2010.
5. Swaim JC, Mansour M, Wydo SM, et al. A retrospective comparison of anesthetic agents in electroconvulsive therapy. J ECT. 2006;22:243-246.
6. Vaidya P, Anderson E, Bobb A, et al. A within-subject
comparison of propofol and methohexital anesthesia for electroconvulsive
therapy. J ECT. 2012;28:14-19.
7. Patel PM, Patel HH, Roth DM. Chapter 19. General
anesthetics and therapeutic gases. In: Brunton LL, Chabner BA, Knollmann
BC, eds. Goodman & Gilman’s The Pharmacological Basis of Therapeutics. 12th ed. New York, NY: McGraw-Hill; 2011. www.accesspharmacy.com/content.aspx?aID=16664636. Accessed August 2, 2013.
8. Geretsegger C, Nickel M, Judendorfer, et al. Propofol
and methohexital as anesthetic agents for electroconvulsive therapy: a
randomized, double-blind comparison of electroconvulsive therapy seizure
quality, therapeutic efficacy, and cognitive performance. J ECT. 2007;23:239-243.
9. Eranti SV, Mogg AJ, Pluck GC, et al. Methohexitone,
propofol and etomidate in electroconvulsive therapy for depression: a
naturalistic comparison study. J Affect Disord. 2009;113:165-171.
10. Kellner C. Lesson from the methohexital shortage. J ECT. 2003;19:127-128.
To comment on this article, contact rdavidson@uspharmacist.com.





