US Pharm. 2024;49(7):HS11-HS16.
ABSTRACT: Severe community-acquired pneumonia (CAP) and acute respiratory distress syndrome (ARDS) are commonly seen in the hospital setting. Both of these diseases have high rates of morbidity and mortality. Corticosteroids may have utility for CAP and ARDS by reducing lung inflammation. Various corticosteroid dosing regimens for severe CAP and ARDS exist, and critical illness–related corticosteroid insufficiency guidelines were updated in early 2024. Pharmacists play an integral role in the management of severe CAP and ARDS, and it is essential for them to be aware of the current guideline updates, the different dosing regimens recommended, and these regimens’ appropriateness for their individual patients.
Patients who are acutely ill may have a dysregulated inflammatory response requiring the use of corticosteroids, which have a broad anti-inflammatory mechanism of action. Corticosteroid treatment has been used in a variety of pulmonary diseases to reduce morbidity and mortality. Although corticosteroids have a known benefit in asthma and other pulmonary diseases, their role remains controversial in many other conditions. The choice of corticosteroid plays a major role in therapy, as the various corticosteroids have different glucocorticoid and mineralocorticoid pharmacodynamic properties. Additionally, the correct choice of corticosteroid and dosing regimen are frequently debated by experts. The routine use of corticosteroids for severe and nonsevere community-acquired pneumonia (CAP) in the inpatient setting was not recommended by the American Thoracic Society (ATS) and Infectious Diseases Society of America (IDSA) guideline in 2019.1 However, more recently, a clinical trial concluded that corticosteroid administration resulted in a lower risk of death in patients with severe CAP.2 Also, the utilization of corticosteroids in the setting of acute respiratory distress syndrome (ARDS) has evolved over the past few decades based on increased data from clinical trials.
In 2008, critical-care medicine and endocrinology experts from the Society of Critical Care Medicine and the European Society of Intensive Care Medicine coined the term critical illness–related corticosteroid insufficiency (CIRCI) to describe a state of systemic inflammation involving dysregulation of the hypothalamic-pituitary-adrenal axis, altered cortisol metabolism, and tissue glucocorticoid resistance.3 In 2017, the guidelines were updated to include recommendations on the use of corticosteroids to treat certain conditions in patients with CIRCI. The newest guideline update was published in early 2024.3 This article will review the use of corticosteroids in patients with severe CAP and ARDS.
Corticosteroid Pharmacology
Corticosteroids are categorized into two main pharmacologic groups: glucocorticoids and mineralocorticoids. Glucocorticoids exhibit numerous physiologic effects, largely regulating metabolism and inflammation and modulating the immune system. Glucocorticoids act via both genomic and nongenomic processes. The genomic effects of glucocorticoids are mediated by the formation of a complex with glucocorticoid receptors. This complex subsequently modulates the expression of genes encoding various inflammatory mediators, such as nuclear factor kappa B and tumor necrosis factor alpha.4 There are three main nongenomic pathways: nongenomic interactions with cytosolic glucocorticoid receptors, interactions with membrane-bound receptors, and nonspecific effects such as interactions with cellular plasma membranes.4 Mineralocorticoids function to regulate sodium and water balance via interactions with mineralocorticoid receptors.5,6
Corticosteroids pertinent to clinical practice include hydrocortisone, prednisone, prednisolone, methylprednisolone, and dexamethasone. Each agent has a unique relative potency profile for both anti-inflammatory and mineralocorticoid activities compared with the endogenous glucocorticoid cortisol. Relative anti-inflammatory potency dose equivalents are as follows: dexamethasone, 0.75 mg; methylprednisolone, 4 mg; prednisone/prednisolone, 5 mg; and hydrocortisone, 20 mg. These equivalents are impacted by the dose of glucocorticoid used; genomic effects predominate at lower doses (e.g., <100 mg prednisone equivalents), whereas nongenomic effects predominate at higher doses, and nongenomic relative efficacies vary.7 Relative mineralocorticoid potencies are as follows: dexamethasone, 0; methylprednisolone, 0.5; prednisone/prednisolone, 0.8; and hydrocortisone, 1.8
Community-Acquired Pneumonia
CAP is a common reason for ICU admission. In 2019 (including the period prior to the start of the COVID-19 pandemic), CAP was the chief cause of infectious disease–related mortality and the second leading cause of disability-adjusted life-years globally.9 Despite the use of appropriate antimicrobials and supportive measures, mortality in patients with severe CAP remains high. In one large study, a pathogen was detected during diagnostic testing in only 38% of patients with radiographic evidence of pneumonia, highlighting the potential difficulty of narrowing treatment for patients with CAP.10 In another study, a higher circulating inflammatory-cytokine level in patients admitted to the ICU was correlated with the presence of bacteremia and the need for mechanical ventilation.11
Corticosteroids have been used in severe CAP to mitigate the inflammatory lung injury. Early administration of glucocorticoids might be beneficial in CAP owing to the glucocorticoid receptor’s mechanism of action. The glucocorticoid receptor is a transcription factor that is expressed in most cells, and it exerts both genomic effects and nongenomic effects.9 During inflammation, as with severe CAP, modifications alter the ability of glucocorticoid receptors to regulate the transcription of inflammatory genes. Corticosteroids administered in the early stage of CAP decrease inflammatory cytokine production, neutrophils, and the recruitment and adhesion of T cells.9
In 2019, the ATS/IDSA guideline did not recommend the routine use of corticosteroids in adults with severe CAP.1 This guidance stemmed from the lack of clinically important endpoints such as mortality, length of stay, and organ failure in randomized, controlled trials (RCTs). One study found a sizable difference in mortality in patients treated with corticosteroids; however, this could not be replicated in other trials, raising the concerns over potential overestimation of true effect.1 At the time of publication, the ATS/IDSA guideline advised that large, multicenter, randomized trials with well-defined inclusion and exclusion criteria were needed to assess the use of corticosteroids in CAP. A 2022 study that evaluated the use of low-dose methylprednisolone in critically ill patients with severe CAP determined that a 20-day regimen of methylprednisolone did not significantly reduce 60-day mortality in these patients.12 However, in a study published in 2023, hydrocortisone significantly reduced the risk of death by day 28 in critically ill patients with severe CAP compared with those who received placebo.2 The differences between these studies might stem from the choice of corticosteroid, as the balance of mineralocorticoids and glucocorticoids differs (as previously discussed). Additionally, the early administration of hydrocortisone (<15 hours from ICU admission to first hydrocortisone dose) may have led to early decreased inflammatory response.
Following on the the heels of the aforementioned trial results, the 2024 CIRCI guidelines recommend administering corticosteroids to adults hospitalized with severe bacterial CAP.3 It is recognized that multiple dosing strategies exist for corticosteroids used to treat severe CAP. TABLE 1 summarizes the potential options.3
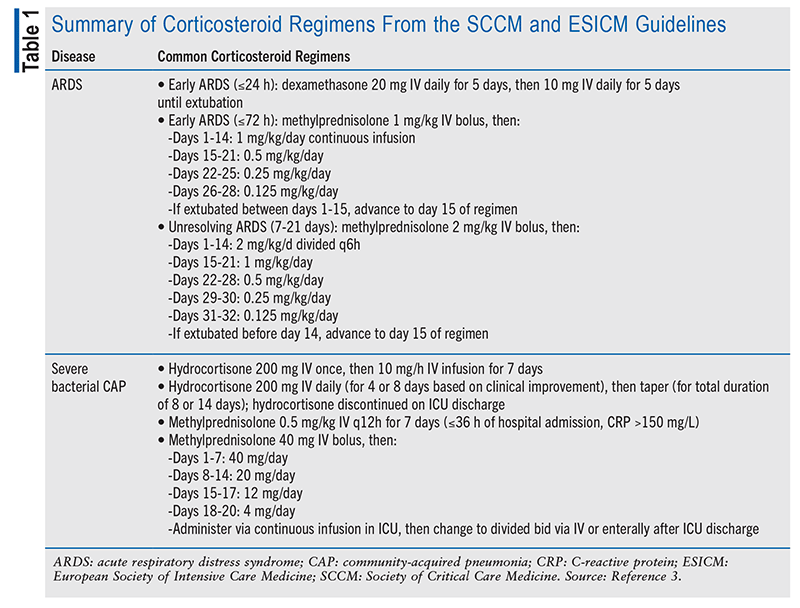
Acute Respiratory Distress Syndrome
ARDS is a heterogenous disease characterized by the development, within 1 week, of a known clinical insult or new or worsening respiratory symptoms; bilateral opacities that are not fully explained by effusions; lobar or lung collapse or nodules; respiratory failure not fully explained by cardiac failure or fluid overload; and impaired oxygenation with an oxygen pressure/fraction of inspired oxygen (P/F) ratio of ≤300 mmHg with a positive end expiratory pressure of ≥5 mmHg according to the Berlin criteria.13 The incidence of ARDS varies significantly across different countries; however, an international study conducted over a 4-week period found that 10% of all ICU patients and 23% of patients on mechanical ventilation met the Berlin definition of ARDS.14 This disease has high rates of morbidity and mortality. Mortality rates were incorporated into the Berlin criteria based on severity: 34.9% for mild (P/F ≤300), 40.3% for moderate (P/F ≤200), and 46.1% for severe ARDS (P/F ≤100).13,15
The pathogenesis of ARDS is driven by dysregulated inflammation and is categorized into three phases: exudative, proliferative, and fibrotic. A large part of the inflammatory processes and insult occurs during the initial exudative phase, which is characterized by immune cell–mediated alveolar damage and perpetuated by cytokine production, neutrophil and macrophage chemotaxis, endothelial activation, and microvascular injury.16 Given the proinflammatory nature of ARDS, the potential role of corticosteroids has been explored extensively, with mixed results. These results were analyzed in a recent ATS clinical practice guideline update that identified 19 RCTs that assessed corticosteroid use in a total of 2,790 patients.17 Based on the pooled analysis, it was concluded that corticosteroids probably decrease mortality and may reduce the duration of mechanical ventilation and hospital length of stay. It also was noted that corticosteroids probably increase the risk of serious hyperglycemia, may increase the risk of gastrointestinal bleeding, and have an uncertain effect on neuromuscular weakness.17
The guideline does not make recommendations for a specific agent or duration, but it suggests that clinicians refer to corticosteroid literature specific to the inciting factor or regimens studied in RCTs for heterogenous ARDS populations (TABLE 1). One notable trial that the 2017 CIRCI guidelines cite for its dosing recommendation for corticosteroids in early ARDS was published in 2007.18,19 The researchers investigated a regimen of methylprednisolone 1 mg/kg/day loading dose followed by 1 mg/kg for 14 days, 0.5 mg/kg for 7 days, 0.25 mg/kg for 3 days, and 0.125 mg/kg for 3 days. This regimen was associated with improved organ function as well as reduced duration of mechanical ventilation and ICU length of stay.18
Subsequent meta-analyses had mixed results regarding the benefit of corticosteroids in ARDS.20-23 Accordingly, investigators continued to explore different dosing regimens and agents, leading to another key trial, which evaluated dexamethasone use in ARDS. A regimen of dexamethasone 20 mg daily for 5 days followed by 10 mg daily for 5 days was found to lead to reduced duration of mechanical ventilation and mortality.24 As discussed earlier, dexamethasone lacks mineralocorticoid activity and may have less impact on fluid retention in a patient population in whom the literature previously demonstrated improved outcomes with a conservative fluid strategy.25 The ATS guideline also notes that corticosteroid initiation in late ARDS (>14 days after onset) may be associated with harm.17 This is based on an RCT in which the initiation of methylprednisolone >2 weeks after onset was associated with increased mortality.26
The Pharmacist’s Role
Pharmacists play an integral role in the management of severe CAP and ARDS. Pharmacists are primed to evaluate the literature and make recommendations as to the corticosteroid type, dosing, and duration. It is essential for pharmacists to be aware of the current guideline updates, the different dosing regimens recommended, and these regimens’ appropriateness for their individual patients.
REFERENCES
1. Metlay JP, Waterer GW, Long AC, et al. Diagnosis and treatment of adults with community-acquired pneumonia. An official clinical practice guideline of the American Thoracic Society and Infectious Diseases Society of America. Am J Respir Crit Care Med. 2019;200(7):e45-e67.
2. Dequin PF, Meziani F, Quenot JP, et al. Hydrocortisone in severe community-acquired pneumonia. N Engl J Med. 2023;388(21):1931-1941.
3. Chaudhuri D, Nei AM, Rochwerg B, et al. 2024 focused update: guidelines on use of corticosteroids in sepsis, acute respiratory distress syndrome, and community-acquired pneumonia. Crit Care Med. 2024;52(5):e219-e233.
4. Sinha A, Bagga A. Pulse steroid therapy. Indian J Pediatr. 2008;75(10):1057-1066.
5. Ericson-Neilsen W, Kaye AD. Steroids: pharmacology, complications, and practice delivery issues. Ochsner J. 2014;14(2):203-207.
6. Hodgens A, Sharman T. Corticosteroids. In: StatPearls [Internet]. Treasure Island, FL: StatPearls Publishing; 2024 Jan-.
7. Buttgereit F, da Silva JAP, Boers M, et al. Standardised nomenclature for glucocorticoid dosages and glucocorticoid treatment regimens: current questions and tentative answers in rheumatology. Ann Rheum Dis. 2002;61(8):718-722.
8. Williams DM. Clinical pharmacology of corticosteroids. Respir Care. 2018;63(6):655-670.
9. Bouras M, Rello J, Roquilly A. Steroids in severe community-acquired pneumonia: dangerous, worthless, or miracle cure? The roller coaster of clinical trials. Anaesth Crit Care Pain Med. 2023;42(4):101253.
10. Jain S, Self WH, Wunderink RG, et al. Community-acquired pneumonia requiring hospitalization among U.S. adults. N Engl J Med. 2015;373(5):415-427.
11. Fernández-Serrano S, Dorca J, Coromines M, et al. Molecular inflammatory responses measured in blood of patients with severe community-acquired pneumonia. Clin Diagn Lab Immunol. 2003;10(5):813-820.
12. Meduri GU, Shih MC, Bridges L, et al. Low-dose methylprednisolone treatment in critically ill patients with severe community-acquired pneumonia. Intensive Care Med. 2022;48(8):1009-1023.
13. ARDS Definition Task Force; Ranieri VM, Rubenfeld GD, Thompson BT, et al. Acute respiratory distress syndrome: the Berlin Definition. JAMA. 2012;307(23):2526-2533.
14. Bellani G, Laffey JG, Pham T, et al. Epidemiology, patterns of care, and mortality for patients with acute respiratory distress syndrome in intensive care units in 50 countries. JAMA. 2016;315(8):788-800.
15. Hendrickson KW, Peltan ID, Brown SM. The epidemiology of acute respiratory distress syndrome before and after coronavirus disease 2019. Crit Care Clin. 2021;37(4):703-716.
16. Gragossian A, Siuba MT. Acute respiratory distress syndrome. Emerg Med Clin North Am. 2022;40(3):459-472.
17. Qadir N, Sahetya S, Munshi L, et al. An update on management of adult patients with acute respiratory distress syndrome: an official American Thoracic Society clinical practice guideline. Am J Respir Crit Care Med. 2024;209(1):24-36.
18. Meduri GU, Golden E, Freire AX, et al. Methylprednisolone infusion in early severe ARDS: results of a randomized controlled trial. Chest. 2007;131(4):954-963.
19. Annane D, Pastores SM, Rochwerg B, et al. Guidelines for the diagnosis and management of critical illness-related corticosteroid insufficiency (CIRCI) in critically ill patients (part I): Society of Critical Care Medicine (SCCM) and European Society of Intensive Care Medicine (ESICM) 2017. Intensive Care Med. 2017;43(12):1751-1763.
20. Hough CL. Steroids for acute respiratory distress syndrome? Clin Chest Med. 2014;35(4):781-795.
21. Peter JV, John P, Graham PL, et al. Corticosteroids in the prevention and treatment of acute respiratory distress syndrome (ARDS) in adults: meta-analysis. BMJ. 2008;336(7651):1006-1009.
22. Tang BMP, Craig JC, Eslick GD, et al. Use of corticosteroids in acute lung injury and acute respiratory distress syndrome: a systematic review and meta-analysis. Crit Care Med. 2009;37(5):1594-1603.
23. Lamontagne F, Briel M, Guyatt GH, et al. Corticosteroid therapy for acute lung injury, acute respiratory distress syndrome, and severe pneumonia: a meta-analysis of randomized controlled trials. J Crit Care. 2010;25(3):420-435.
24. Villar J, Ferrando C, Martínez D, et al. Dexamethasone treatment for the acute respiratory distress syndrome: a multicentre, randomised controlled trial. Lancet Respir Med. 2020;8(3):267-276.
25. Wiedemann HP, Wheeler AP, Bernard GR, et al. Comparison of two fluid-management strategies in acute lung injury. N Engl J Med. 2006;354(24):2564-2575.
26. Steinberg KP, Hudson LD, Goodman RB, et al. Efficacy and safety of corticosteroids for persistent acute respiratory distress syndrome. N Engl J Med. 2006;354(16):1671-1684.
The content contained in this article is for informational purposes only. The content is not intended to be a substitute for professional advice. Reliance on any information provided in this article is solely at your own risk.
To comment on this article, contact rdavidson@uspharmacist.com.






