US Pharm. 2024;49(10):39-46,
ABSTRACT: The adverse effect (AE) profile of the two mRNA COVID-19 vaccines (Pfizer and Moderna) and the recombinant protein COVID-19 vaccine (Novavax) that are currently available differ widely. Myocarditis/pericarditis are widely recognized AEs of the mRNA vaccines. There are conflicting data on the relationship between mRNA COVID-19 vaccines and Bell’s palsy. Guillain-Barré syndrome does not appear to be an AE of mRNA COVID-19 vaccines. Anaphylaxis and lymphadenopathy are systemic immunological AEs associated with the mRNA COVID-19 vaccines. Novavax COVID-19 vaccine–related symptoms involve more traditional systemic reactions. Pharmacists can play a major role in promoting public health by dispelling misinformation about COVID-19 vaccine safety, fulfilling their role as mandatory reporters of AEs, and educating the public about the proven benefits of vaccination.
As of September 5, 2024, three COVID-19 vaccines were available in the United States.1 Two mRNA COVID-19 vaccines were available: Moderna COVID-19 Vaccine (2024-2025 Formula) and Spikevax. Moderna COVID-19 Vaccine (2024–2025 Formula) is authorized for children aged 6 months to 11 years; SPIKEVAX is the licensed Moderna product for people aged 12 years and older. The FDA refers to these vaccines as 2024–2025 Moderna COVID-19 Vaccine.
Pfizer-BioNTech COVID-19 Vaccine (2024–2025 Formula) is authorized for children aged 6 months to 11 years; COMIRNATY is the licensed Pfizer-BioNTech product for people aged 12 years and older. The FDA refers to these vaccines as 2024–2025 Pfizer-BioNTech COVID-19 Vaccine.
Novavax COVID-19 Vaccine, Adjuvanted (2024–2025 Formula), a protein subunit vaccine, is authorized for people aged 12 years and older. It is hereafter referred to as 2024–2025 Novavax COVID-19 Vaccine. (The 2023-2024 Moderna, Novavax, and Pfizer-BioNTech COVID-19 vaccines are no longer recommended and should not be used.)
Information discussed in this article is based on the
prescribing information/emergency use authorization (EUA) for the 2023-2024 COVID-19 vaccines.2-4 Janssen’s COVID-19 vaccine, which was an adenovirus vector vaccine, had its EUA revoked on June 1, 2023, and is not discussed.5 This article will provide an update on safety data for the COVID-19 vaccines, excluding Janssen’s. The latest recommendations of the Advisory Committee on Immunization Practices in the U.S. for 2024-25 were published in the September 10, 2024, early release issue of Morbidity and Mortality Weekly Report.
CDC Vaccine Safety and Monitoring Website
The CDC’s website provides information on COVID-19 vaccine safety, healthcare provider and parent/caregiver information on vaccine safety, and facts about vaccine safety. The website also contains links to the U.S. Vaccine Adverse Event Reporting System (VAERS), the Vaccine Safety Datalink, V-safe, the Clinical Immunization Safety Assessment project, and the COVID-19 Vaccine Pregnancy Registry.
VAERS serves as the nation’s early warning system to monitor for potential vaccine safety problems. Pharmacists have a professional obligation to file a report if an adverse event (AE) to a vaccination is suspected (see SIDEBAR 1).6,7

The 2023-2024 updated COVID-19 vaccines are monitored by V-safe, the safety monitoring system for vaccine recipient reporting. The COVID-19 Vaccine Pregnancy Registry is for individuals who enrolled in V-safe and reported receiving a COVID-19 vaccine during pregnancy or within 30 days before their last menstrual period before the pregnancy.
VAERS data and deidentified reports are available to the public on the VAERS and CDC Wide-ranging ONline Data for Epidemiologic Research (WONDER) websites. It is an easy-to-use online system that makes the information resources of the CDC available to public health professionals and the public.8 However, it is important that pharmacists understand the limitations of the VAERS data available through WONDER. VAERS has issued several caveats, including that its data cannot be used to determine if a vaccine caused or contributed to an AE or illness. It warns that the number of reports alone cannot be interpreted as evidence of a causal association between a vaccine and an AE. The VAERS data may be incomplete, inaccurate, coincidental, unverifiable, or in some cases, deliberately biased or misleading despite being subject to a fine and imprisonment under federal law. Follow-up information is not obtained for every report, and additional information, if obtained, is not included in the WONDER database as it only allows for initial event reporting. VAERS data do not represent all known safety information.
The VAERS program advises that its data be considered in the context of other scientific information, as the VAERS program is designed to rapidly detect “safety signals” or unusual or unexpected patterns of AEs associated with a particular vaccine.8
COVID-19 Vaccine Safety Updates
Vaccines are often available and/or used in combination, making association between AEs and a particular vaccine component difficult to assess. Further, the literature sometimes does not indicate the trade name of a vaccine, nor may it indicate its availability in the U.S. Additional difficulties in reviewing statistics on AEs to COVID-19 vaccines include that they have been developed using various platforms and with several names for the same vaccine. Patients may have received different brands for their original vaccine and subsequent boosters, which makes data collection and establishing correlation complicated.9
Based on prescribing information (PI), adverse reactions that are most commonly associated with Pfizer’s mRNA COVID-19 vaccine (PmRNACV) include pain at the injection site (up to 90.5%), fatigue (up to 77.5%), headache (up to 75.5%), chills (up to 49.2%), muscle pain (up to 45.5%), joint pain (up to 27.5%), fever (up to 24.3%), injection-site swelling (up to 11.8%), and injection-site redness (up to 10.4%).2
The AEs most often seen with Moderna’s mRNA COVID-19 vaccine (MmRNVACV) are reported by age in the PI. The PI for Spikevax includes data on AEs for those aged 12 to 17 years, 18 to 64 years, and >65 years. Pain at the injection site, axillary swelling/tenderness, and nausea/vomiting were reported as most common in those aged 12 to 17 years, whereas fatigue, headache, myalgia, arthralgia, and chills were seen more often in those aged 18 to 64 years. The most common AEs for those aged 65 years and older were pain at t injection site, fatigue, myalgia, headache, and arthralgia.3
Novavax’s COVID-19 vaccine AE profile includes injection-site pain/tenderness, fatigue/malaise, muscle pain, headache, joint pain, nausea/vomiting, and injection-site reactions.4
The number of COVID-19 vaccinations administered in the U.S. differ based on manufacturer. According to the website Our World in Data, as of March 22, 2023, the last date for which data are available for the U.S., 401.69 million, 251.85 million, 18.99 million, and 83,047 doses of PmRNACV, MmRNVACV, Johnson & Johnson, and Novavax’s COVID-19 vaccines, respectively, had been administered.10
The CDC has a webpage highlighting selected AEs reported after COVID-19 vaccination, which include anaphylaxis, death, Guillain-Barré syndrome (GBS), myocarditis, pericarditis, and thrombosis with thrombocytopenia syndrome.11
Due to the global nature of the COVID-19 pandemic, the International Network of Special Immunization Services was formed to monitor for AEs of special interest (AESIs), to identify the pathogenesis of AESIs, and to assist with future vaccine development.12
The following is an update of selected serious reactions that have been associated with COVID-19 vaccines. It is important to point out that causality has not been established. The following is based on a review of the literature using Medical Subject Headings.
Serious Cardiovascular/Hematological Reactions
Myocarditis or Pericarditis: Myocarditis is defined as inflammation of the myocardium with or without necrosis, whereas pericarditis is inflammation of the pericardium. The presence of both acute myocarditis and pericarditis is called myopericarditis or perimyocarditis.9
A definitive diagnosis of myocarditis requires that an endomyocardial biopsy be performed, which was not typically done during the pandemic. A diagnosis is based on clinical presentation and laboratory data, but there is controversy regarding the appropriateness of using troponin levels in diagnosing myocarditis as they are unreliable biomarkers for myocarditis and may only identify severe or persistent cases.9,13 Therefore, most cases of vaccine-induced myocarditis are considered clinically suspected or probable cases.9
Numerous studies have reported on the development of myocarditis or pericarditis in relation to the use of COVID-19 mRNA vaccines.9,14-80
The mRNA vaccines’ PI postmarketing data for authorized or approved mRNA COVID-19 vaccines cite increased risks of myocarditis and pericarditis, particularly within the first week following vaccination. Although some cases required intensive-care support, available data from short-term follow-up suggest that most individuals have had resolution of symptoms with conservative management. Information is not yet available about potential long-term sequelae.2,3
MmRNVACV’s PI provides more information on myocarditis/pericarditis and how the company is monitoring for the potential AEs.3
The Global Vaccine Data Network (GVDN) is a vaccine research network involving 31 sites in 26 countries on six continents and has included data on >300 million people. Retrospective observational data from this network of >99 million persons followed for 42 days post injection identified a statistically significant, increased prioritized signal fulfilling the safety threshold for myocarditis for the first, second, and third doses of both mRNA COVID-19 vaccines. The highest observed to expected (O/E) ratio for the occurrence of myocarditis was following the first and second dose of the MmRNVACV.81
Overall, COVID-19 mRNA vaccine–associated myocarditis accounted for 68% of all cases of vaccine-associated myocarditis or pericarditis reported to VAERS between January 1, 2021, and July 20, 2021.
AEs to the MmRNVACV from January 1, 2021, through October 27, 2022, accounted for 1,942 AEs or 0.28% of all adverse reactions reported to VAERS. These adverse reactions included myocarditis (25.18%, 489 reports); acute myocardial infarction (24.46%, 475 reports); bradycardia (14.93%, 290 reports); and pericarditis (14.47%, 281 reports).9,82
For PmRNACV, the observed risk of myocarditis is highest in males aged 12 to 17 years, whereas for MmRNVACV, the observed risk is highest in males aged 18 to 24 years.2,3
Analysis of data for adolescents aged 12 to 17 years from VigiBase, the World Health Organization’s global Individual Case Safety Report database, found a statistically significantly elevated adjusted reporting odds ratio (OR) of 19.61 (95% CI 14.05-27.39) for myocarditis/pericarditis for both COVID-19 mRNA vaccines. These accounted for over 95% of all COVID-19 vaccinations.83
The incidence of pericarditis or myocarditis after the PmRNACV in adolescent patients (median age 15 years) presenting to the emergency department with cardiovascular symptoms was found to be approximately 7%.20 Hospitalization rates for myocarditis/pericarditis vary, with reports of up to 87%.39
Risk factors for pediatric intensive unit admission for myocarditis are abnormal electrocardiographic findings and abnormal serum troponin level in the pediatric emergency department.15,20
An increased rate of reports among individuals aged 16 to 17 years was seen when the second dose of PmRNACV was administered with a short (i.e., <30 days) interdose interval (21.3 per 100,000; 95%CI, 11.0-37.2).21 Other reports have found that males aged 15 to 24 years are at highest risk of developing myocarditis following the second COVID-19 mRNA vaccine.37
Children aged 5 to 11 years appear to be at a lower risk of developing myocarditis/pericarditis than adolescents aged 12 to 15 years.30
A compendium that reviewed world literature on COVID-19 and myocarditis/pericarditis found that the incidence of these conditions varied greatly depending on the vaccine type and how many doses were administered. The highest rates of myocarditis/pericarditis were reported for MmRNVACV, with an overall incidence of about 10/100,000 and around 50/100,000 in men aged <40 years. There was consensus among the reports that those at greatest risk of developing myocarditis were men aged between 12 and 39 years; an elevated rate of vaccine-associated myocarditis is not seen in those aged >50 years; however, pericarditis is most often seen in men in this group.9
Myocarditis is most commonly observed following the second COVID-19 mRNA vaccine, although it also can occur after the first dose.14,15,17,20,21,39 Direct head-to-head comparisons and epidemiological studies in both males and females found that rates of myocarditis and pericarditis after either dose of MmRNVACV was modestly higher than for the PmRNACV during the 0 to 7 days post vaccination.29,32,34,37,39,62 Most cases of COVID-19–related myocarditis are transient and self-limiting.25 However, cardiogenic shock requiring mechanical circulatory support has been reported.28 More patients were hospitalized and treated in the intensive care unit for vaccine-related myocarditis (95% hospitalized and 10% in the intensive care unit) than for vaccine-related pericarditis (35% hospitalized and 3% in intensive care).9
Residual effects on late gadolinium enhancement studies may indicate the presence of fibrotic changes. These changes were observed following the resolution of myocardial edema/acute inflammation. However, the significance of these findings is unclear.35,84-86
Among the proposed mechanisms for COVID-19 mRNA vaccine–induced myocarditis are hyperimmune or inflammatory response between the spike protein of SARS-CoV-2 and cardiac myosin; upregulation of inflammatory cytokines and lymphocytes resulting in tissue damage/cytokine storm from the immune response to the vaccine and bystander activation; antibodies directed against interleukin (IL)-1RA; serum sickness; eosinophilic myocarditis; hypersensitivity to vaccine vehicle components (e.g., polyethylene glycol [PEG] and tromethamine, lipid nanoparticle sheath); low residual levels of double-strand RNA; hyperviscosity; and exercise-induced secretion of proinflammatory IL-6. Differences in sex steroid hormones may also play a role.9,34
SARS-CoV-2 infection is associated with the development of ventricular arrhythmias, acute coronary syndromes with obstructive coronary artery disease such as myocardial infarction, thromboembolic syndromes including stroke, acute myocardial damage with elevated troponin levels without evidence of coronary artery disease (i.e., myocarditis), and heart failure.9
Others have also found a greater risk of myocarditis following infection with SARS-CoV-2 than with the COVID-19 mRNA vaccines.75 The risk may be ≥15-fold for developing myocarditis from SARS-CoV-2 infection compared with other causes, including the COVID-19 mRNA vaccine.9
A systematic review of AE reporting data from both Europe and the U.S., performed along with a review of the scientific literature, found a mortality rate of 0.22% (n = 30) among 13,571 myocarditis or pericarditis events reported for COVID-19 mRNA vaccines. Fatalities from myocarditis, pericarditis, and myopericarditis were more common in older adults (myocarditis, median age 60 years; pericarditis, median age 71 years; and myopericarditis, which occurred in two patients aged 55 and 83 years).38
Despite these concerns, postvaccination myocarditis is associated with a 92% lower mortality risk (adjusted hazard ratio [HR]: 0.08; 95% CI 0.01-0.57) compared with postviral myocarditis.24
A French cohort study conducted from December 27, 2020, to June 30, 2022, followed 4,635 patients who were hospitalized with myocarditis associated with the mRNA COVID-19 vaccines (i.e., vaccine administered within the previous 7 days of diagnosis, N = 558); patients with post–COVID-19 myocarditis (i.e., patient developed myocarditis within 30 days of SARS-CoV-2 infection, N = 298); and patients with conventional myocarditis (N = 3779). While the results cannot be viewed as causal, after 18 months of follow-up, investigators found that unlike those with post–COVID-19 myocarditis (weighted HR 1.04; 94% CI 0.70-1.52), those with vaccine-associated myocarditis (weighted HR 0.55; 95% CI 0.36-0.86) had fewer hospital readmissions for myopericarditis, other cardiovascular events, or all-cause death as a composite outcome compared with those who experienced conventional myocarditis.87
The CDC has published considerations related to myocarditis and pericarditis after vaccination, including for vaccination of individuals with a history of myocarditis or pericarditis.88
Pharmacists should counsel patients and/or parents/caregivers to monitor for chest pain, shortness of breath, or feelings of having a fast-beating, fluttering, or pounding heart, especially if these symptoms occur during the 2 weeks following a dose of the vaccine.88
Central Nervous System Reactions
Bell’s Palsy: Retrospective observational data from the GVDN, VAERS data, a self-controlled study using Medicare data on COVID-19 mRNA booster doses, and a real-world study of >200,000 vaccinated patients did not observe any significant signals for facial paralysis or Bell’s palsy (BP).81,89-91 However, others have found that the risk of BP is more frequently observed than expected based on vaccine brand, sex, and age.92
In a systematic review and meta-analysis, the odds of developing BP were significantly greater with SARS-CoV-2 vaccines (mRNA and viral vector) in randomized, controlled studies compared with placebo. In observational studies, however, those who received mRNA COVID-19 vaccines were not at greater risk of developing BP than those who were not vaccinated.
As a result of a strong association between the SARS-CoV-2 vaccine and BP, in four randomized, controlled trials investigators concluded that the development of BP was related to vaccine exposure. However, SARS-CoV-2 infection was linked with a 3.23-fold increased risk of BP compared with SARS-CoV-2 vaccinations (relative risk 3.23; 95% CI 1.57-6.62).93
A nested case-control and self-controlled case series study found a significant increase in BP in those who received PmRNACV (adjusted OR 1.543; 95% CI 1.123-2.121), resulting in 1.112 excess events per 100,000 recipients of two doses of the vaccine. The increased risk of BP was greatest during the first 2 weeks following vaccine administration.94
Guillain-Barré Syndrome: GBS is an acute, inflammatory polyneuropathy that is due to an infection or adenoviral vector–based vaccines.95 Numerous sources did not identify a correlation between GBS and mRNA COVID-19 vaccines.81,83,96 In fact, one study found that the PmRNACV was associated with a reduced risk of developing GBS. Having had COVID-19 increases the risk of GBS.97
Systemic Immunologic Reactions
Anaphylaxis: There is no uniformly accepted definition of anaphylaxis following immunizations.98 Although anaphylaxis is mentioned in the PIs for both the PmRNACV and MmRNVACV as a contraindication for administration, no details are provided.2,3 The literature has reported the rate of anaphylaxis from the PmRNACV and MmRNVACV as 2.5 to 11 cases per million doses.1,99
In a population-based study conducted from December 16, 2020, to March 11, 2021, focusing on the frequency, severity, and risk factors associated with treated acute-onset hypersensitivity reactions from first and second-dose exposures to COVID-19 mRNA vaccines, researchers found that the calculated rate of anaphylaxis was 3.29 per million doses administered (0.00033%), with hypersensitivity reactions being approximately 2 times more likely to occur with first doses than second doses of the vaccine. This study involved over 215,000 persons who received either the PmRNACV or MmRNVAV. Risk factors for hypersensitivity reactions included female gender (86.9%), younger age, having underlying multiple drug intolerance syndrome (MDIS), and having a clinical history with known risk factors for MDIS, including a previously reported vaccine-associated adverse reaction.100
While anaphylaxis is rare, it tends to be more severe in older adults. The Allergic Rhinitis and its Impact on Asthma, European Academy of Allergy and Clinical Immunology, and European Geriatric Medicine Society Working Group has published an algorithm for the management of anaphylaxis due to COVID-19 vaccines in older adults.101 Analysis of data of adolescents aged 12 to 17 years from VigiBase did not find a correlation between the COVID-19 vaccines and anaphylaxis.83
It has been postulated that COVID mRNA vaccine hypersensitivity may be secondary to PEG found in the mRNA COVID-19 vaccines, which is used as a stabilizer to maintain the colloidal stability of the nanoparticles in biological fluids and to reduce their uptake by filter organs. PEG is thought to mediate immunoglobulin E (IgE)–mediated hypersensitivity reactions. It is advisable to avoid COVID-19 mRNA vaccines in patients with severe PEG allergies, although this is not mentioned in the PI.102-105
One study involving 20 patients who experienced anaphylaxis following an mRNA COVID-19 injection, however, found that the presence of preexisting anti-PEG IgE antibodies was not a predominant mechanism behind cases of mRNA COVID-19 vaccine–related anaphylaxis, although the authors conceded that they cannot exclude typical anti–PEG IgE-mediated type 1 hypersensitivity reactions as a mechanism for mRNA COVID-19 vaccine anaphylaxis.106
Nonetheless, others have supported the possible causation between PEG allergy and anaphylaxis to the mRNA COVID-19 vaccines.103-105
A systematic review and meta-analysis were conducted to assess the risk of a severe immediate allergic reaction (i.e., anaphylaxis or requiring the need of injectable epinephrine) to a second dose of a COVID-19 mRNA vaccine in persons who had an immediate allergic reaction of any severity to the first dose of vaccine. Among 78 patients who had experienced a severe immediate allergic reaction (e.g., anaphylaxis) to the first COVID-19 mRNA injection, upon revaccination only four of these patients experienced a severe immediate allergic reaction (e.g., anaphylaxis) again.107 The CDC states, however, that giving the same type of COVID-19 vaccine is contraindicated after a severe allergic reaction (e.g., anaphylaxis) from a previous dose or to a component of the COVID-19 vaccine. It is recommended to administer an alternative COVID-19 vaccine type.108
Lymphadenopathy: Enlargement of cervical and axillary lymph nodes (lymphadenopathy) on the ipsilateral side of the COVID-19 mRNA injection site is a common AE associated with PmRNACV and MmRNVACV. This can lead to diagnostic confusion regarding the presence of metastases, the performance of unnecessary tests (e.g., biopsies), and patient distress.109-112
The exact mechanism for the lymphadenopathy is unknown, but it is thought to be due to an increased immune response following vaccination that results in a localized inflammatory response in the area around the injection site.109
While the PIs of both mRNA COVID-19 vaccines do not provide much detail, Novavax’s information states that the incidence of lymphadenopathy-related reactions is 0.3% among vaccine recipients versus 0.1% placebo recipients and that injection-site pruritus occurs in 0.1% vaccine recipients versus 0.0% placebo recipients. Additionally, lymphadenopathy-related reactions included lymphadenopathy, lymphadenitis, lymph node pain, and axillary pain. All lymphadenopathy-related reactions occurred in participants aged 18 to 64 years, according to Novavax’s EUA.2-4
Lymphadenopathy most commonly occurs in those aged <50 years; following an mRNA vaccine; after the first dose of the vaccine; and when administered within 4 weeks of the diagnostic ultrasound.113 Lymphadenopathy develops within 2 weeks after vaccination following the second dose of either mRNA COVID-19 vaccine. It occurs more commonly following the MmRNVACV (19%).114
In general, the mean time to resolution of lymphadenopathy on ultrasound is shorter following a booster vaccine (102 days) compared with the first dose of the initial series (129 days).115 Lymphadenopathy associated with PmRNACV resolved more quickly than that associated with MmRNVACV (117 vs. 139 days, respectively, after the first dose). Following up at least 12 weeks after vaccine-related lymphadenopathy is suspected is recommended.116
In patients with a history of cancer, guidelines recommend documenting the vaccination date and laterality and injecting into the contralateral side of the known malignancy. Imaging studies for urgent clinical indications or required-to-treat newly diagnosed breast cancer should not be postponed.117
Lymphadenopathy associated with COVID-19 vaccines is not thought to be harmful and should not be a reason to withhold future vaccinations. Imaging such as mammography should be performed before or 4 to 12 weeks following vaccination.109
Novavax COVID-19 Recombinant Protein Vaccine
On July 13, 2022, the FDA issued an EUA for the NVX-CoV2373 (Novavax) COVID-19 vaccine.118 There are limited data on AEs associated with Novavax vaccine in the literature; however, it appears to be well tolerated.119-130
One clinical trial of more than 4,400 participants found that the most common AEs associated with the vaccine for both the first and second doses were headache (20%-25%), muscle pain (17%-20%), and fatigue (12%-16%), with a duration of <3 days. Severe local AEs were uncommon and occurred most often after the second dose (4% Novavax vs. 1% placebo).120 There was a similar to slightly higher frequency of severe AEs between the vaccine and placebo groups.121,131
Rare cases of myocarditis have been reported with Novavax.121,128,132 There are an estimated 1,805 COVID-19 cases prevented per 100,000 vaccinated, compared with 5.3 excess cases of myocarditis/pericarditis per 100,000 vaccinated.133 Despite Novavax’s better safety profile, it produces a lower antibody response to SAR-CoV-2 and is associated with a higher incidence of new COVID-19 infections.130
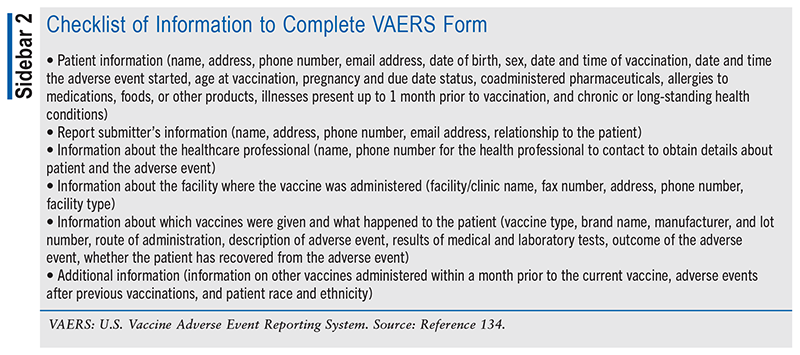
The American Pharmacists Association and the American Society of Health-System Pharmacists have both issued guidelines supporting the pharmacist’s role in monitoring for and reporting AEs associated with vaccinations (see SIDEBAR 2.)6,134,135
Conclusion
There is much misinformation about the safety profile of COVID-19 vaccines available online and in social media. Pharmacists can play a major role in promoting public health by dispelling misinformation about COVID-19 vaccine safety and educating the public about the proven benefits of vaccination.
REFERENCES
- CDC. Interim clinical considerations for use of COVID-19 vaccines in the United States. https://www.cdc.gov/vaccines/covid-19/clinical-considerations/interim-considerations-us.html#:~:text=Moderna%20COVID%2D19%20Vaccine%20(2023,ages%2012%20years%20and%20older. Accessed September 5, 2024.
- Comirnaty (COVID-19 vaccine, mRNA) injection, suspension prescribing information. New York, NY: Pfizer Laboratories; July 1, 2024. https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=48c86164-de07-4041-b9dc-f2b5744714e5. Accessed August 5, 2024.
- Spikevax (COVID-19 vaccine, mRNA) suspension. Princeton, NJ; Moderna US; April 30, 2024. https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=f96b315c-fa57-4876-a7e5-a9b584d8e6e6. Accessed August 5, 2024.
- Novavax, Inc. Novavax EUA Fact Sheet. October 3, 2023. https://novavax.widen.net/s/9tkdmzh9z6/us-eua-fact-sheet-hcp Accessed August 5, 2024.
- UpToDate LexiDrug app. LexiDrugs/COVID-10 Vaccine (Adenovirus Vector). Accessed August 12, 2024.
- American Pharmacists Association. Guidelines for pharmacy-based immunization advocacy and administration. January 26, 2019. https://aphanet.pharmacist.com/sites/default/files/files/Guidelines_for_Pharmacy_Based_IMZ_Advocacy_Approved_Jan_26_2019.pdf. Accessed June 29, 2024.
- U.S. Department of Health and Human Services. VAERS home. https://vaers.hhs.gov/reportevent.html. Accessed September 20, 2024.
- CDC. CDC WONDER. https://wonder.cdc.gov/vaers.html. Accessed June 29, 2024.
- Fairweather D, Beetler DJ, Di Florio DN, et al. COVID-19, myocarditis and pericarditis. Circ Res. 2023;132(10):1302-1319.
- Our World in Data. Coronavirus (COVID-19) vaccinations. https://ourworldindata.org/covid-vaccinations?country=~USA. Accessed August 14, 2024.
- CDC. Selected adverse events reported after COVID-19 vaccination. Updated September 12, 2023. https://www.cdc.gov/coronavirus/2019-ncov/vaccines/safety/adverse-events.html. Accessed June 29, 2024.
- Top KA, Chen RT, Levy O, et al. Advancing the science of vaccine safety during the Coronavirus Disease 2019 (COVID-19) pandemic and beyond: launching an international network of special immunization services. Clin Infect Dis. 2022;75(Suppl 1):S11-S17.
- Manno EC, Amodio D, Cotugno N, et al. Higher troponin levels on admission are associated with persistent cardiac magnetic resonance lesions in children developing myocarditis after mRNA-based COVID-19 vaccination. Pediatr Infect Dis J. 2023;42(2):166-171.
- Boker LK, Fluss R, Dichtiar R, et al. Pfizer COVID19 vaccine is not associated with acute cardiovascular events excluding myocarditis―a national self-controlled case series study. Isr J Health Policy Res. 2024;13(1):23.
- Yen CW, Lee J, Chang YT, et al. BNT162b2 immunization-related myocarditis in adolescents and consequent hospitalization: report from a medical center. Pediatr Neonatol. 2023;64(6):659-666.
- Walton M, Pletzer V, Teunissen T, et al. Adverse events following the BNT162b2 mRNA COVID-19 vaccine (Pfizer-BioNTech) in Aotearoa New Zealand. Drug Saf. 2023;46(9):867-879.
- Ho JSY, Sia CH, Ngiam JN, et al. A review of COVID-19 vaccination and the reported cardiac manifestations. Singapore Med J. 2023;64(9):543-549.
- Hu M, Wong HL, Feng Y, et al. Safety of the BNT162b2 COVID-19 vaccine in children aged 5 to 17 years. JAMA Pediatr. 2023;177(7):710-717.
- Hesse EM, Hause A, Myers T, et al; MIS-C Review Group. COVID-19 vaccine safety first year findings in adolescents. Pediatrics. 2023;151(5):e2022060295.
- Liao YF, Tseng WC, Wang JK, et al. Management of cardiovascular symptoms after Pfizer-BioNTech COVID-19 vaccine in teenagers in the emergency department. J Formos Med Assoc. 2023;122(8):699-706.
- Buchan SA, Alley S, Seo CY, et al. Myocarditis or pericarditis events after BNT162b2 vaccination in individuals aged 12 to 17 years in Ontario, Canada. JAMA Pediatr. 2023;177(4):410-418.
- Knudsen B, Prasad V. COVID-19 vaccine induced myocarditis in young males: a systematic review. Eur J Clin Invest. 2023;53(4):e13947.
- Piché-Renaud PP, Morris SK, Top KA. A narrative review of vaccine pharmacovigilance during mass vaccination campaigns: focus on myocarditis and pericarditis after COVID-19 mRNA vaccination. Br J Clin Pharmacol. 2023;89(3):967-981.
- Lai FTT, Chan EWW, Huang L, et al. Prognosis of myocarditis developing after mRNA COVID-19 vaccination compared with viral myocarditis. J Am Coll Cardiol. 2022;80(24):2255-2265.
- Furqan M, Chawla S, Majid M, et al. COVID-19 vaccine-related myocardial and pericardial inflammation. Curr Cardiol Rep. 2022;24(12):2031-2041.
- Pareek M, Steele J, Asnes J, et al. Short-term outcomes after myopericarditis related to COVID-19 vaccination. JACC Cardiovasc Imaging. 2022;15(11):2002-2005.
- Kracalik I, Oster ME, Broder KR, et al; Myocarditis Outcomes After mRNA COVID-19 Vaccination Investigators and the CDC COVID-19 Response Team. Outcomes at least 90 days since onset of myocarditis after mRNA COVID-19 vaccination in adolescents and young adults in the USA: a follow-up surveillance study. Lancet Child Adolesc Health. 2022;6(11):788-798.
- Ilonze OJ, Guglin ME. Myocarditis following COVID-19 vaccination in adolescents and adults: a cumulative experience of 2021. Heart Fail Rev. 2022;27(6):2033-2043.
- Goddard K, Lewis N, Fireman B, et al. Risk of myocarditis and pericarditis following BNT162b2 and mRNA-1273 COVID-19 vaccination. Vaccine. 2022;40(35):5153-5159.
- Hause AM, Shay DK, Klein NP, et al. Safety of COVID-19 vaccination in United States children ages 5 to 11 years. Pediatrics. 2022;150(2):e2022057313.
- Keshavarz P, Yazdanpanah F, Emad M, et al. Myocarditis following COVID-19 vaccination: cardiac imaging findings in 118 studies. Tomography. 2022;8(4):1959-1973.
- Abraham N, Spruin S, Rossi T, et al. Myocarditis and/or pericarditis risk after mRNA COVID-19 vaccination: a Canadian head to head comparison of BNT162b2 and mRNA-1273 vaccines. Vaccine. 2022;40(32):4663-4671.
- Shiyovich A, Witberg G, Aviv Y, et al. Myocarditis following COVID-19 vaccination: magnetic resonance imaging study. Eur Heart J Cardiovasc Imaging. 2022;23(8):1075-1082.
- Pillay J, Gaudet L, Wingert A, et al. Incidence, risk factors, natural history, and hypothesised mechanisms of myocarditis and pericarditis following covid-19 vaccination: living evidence syntheses and review. BMJ. 2022;378:e069445.
- Hadley SM, Prakash A, Baker AL, et al. Follow-up cardiac magnetic resonance in children with vaccine-associated myocarditis. Eur J Pediatr. 2022;181(7):2879-2883.
- Baumeier C, Aleshcheva G, Harms D, et al. Intramyocardial inflammation after COVID-19 vaccination: an endomyocardial biopsy-proven case series. Int J Mol Sci. 2022;23(13):6940.
- Karlstad Ø, Hovi P, Husby A, et al. SARS-CoV-2 vaccination and myocarditis in a Nordic cohort study of 23 million residents. JAMA Cardiol. 2022;7(6):600-612.
- Lane S, Yeomans A, Shakir S. Reports of myocarditis and pericarditis following mRNA COVID-19 vaccination: a systematic review of spontaneously reported data from the UK, Europe and the USA and of the scientific literature. BMJ Open. 2022;12(5):e059223.
- Krug A, Stevenson J, Høeg TB. BNT162b2 vaccine-associated myo/pericarditis in adolescents: a stratified risk-benefit analysis. Eur J Clin Invest. 2022;52(5):e13759.
- Cordero A, Cazorla D, Escribano D, et al. Myocarditis after RNA-based vaccines for coronavirus. Int J Cardiol. 2022;353:131-134.
- Truong DT, Dionne A, Muniz JC, et al. Clinically suspected myocarditis temporally related to COVID-19 vaccination in adolescents and young adults: suspected myocarditis after COVID-19 vaccination. Circulation. 2022;145(5):345-356.
- Nygaard U, Holm M, Bohnstedt C, et al. Population-based incidence of myopericarditis after COVID-19 vaccination in Danish adolescents. Pediatr Infect Dis J. 2022;41(1):e25-e28.
- Witberg G, Barda N, Hoss S, et al. Myocarditis after Covid-19 vaccination in a large health care organization. N Engl J Med. 2021;385(23):2132-2139.
- Dionne A, Sperotto F, Chamberlain S, et al. Association of myocarditis with BNT162b2 messenger RNA COVID-19 vaccine in a case series of children. JAMA Cardiol. 2021;6(12):1446-1450.
- Watkins K, Griffin G, Septaric K, Simon EL. Myocarditis after BNT162b2 vaccination in a healthy male. Am J Emerg Med. 2021;50:815.e1-815.e2.
- Schmitt P, Demoulin R, Poyet R, et al. Acute myocarditis after COVID-19 vaccination: a case report. Rev Med Interne. 2021;42(11):797-800.
- Visclosky T, Theyyunni N, Klekowski N, Bradin S. Myocarditis following mRNA COVID-19 vaccine. Pediatr Emerg Care. 2021;37(11):583-584.
- Das BB, Kohli U, Ramachandran P, et al. Myopericarditis after messenger RNA Coronavirus Disease 2019 vaccination in adolescents 12 to 18 years of age. J Pediatr. 2021;238:26-32.e1.
- Schauer J, Buddhe S, Colyer J, et al. Myopericarditis after the Pfizer messenger ribonucleic acid coronavirus disease vaccine in adolescents. J Pediatr. 2021;238:317-320.
- Kim D, Choi JH, Jang JY, et al. A case report for myopericarditis after BNT162b2 COVID-19 mRNA vaccination in a Korean young male. J Korean Med Sci. 2021;36(39):e277.
- Levin D, Shimon G, Fadlon-Derai M, et al. Myocarditis following COVID-19 vaccination―a case series. Vaccine. 2021;39(42):6195-6200.
- Walters CG, Jaiswal DD, Hu TX, Kim SS. Myopericarditis in a young adult secondary to COVID-19 vaccination. Methodist Debakey Cardiovasc J. 2021;17(3):13-17.
- Marshall M, Ferguson ID, Lewis P, et al. Symptomatic acute myocarditis in 7 adolescents after Pfizer-BioNTech COVID-19 vaccination. Pediatrics. 2021;148(3):e2021052478.
- O’Leary ST, Maldonado YA. Myocarditis after SARS-CoV-2 vaccination: true, true, and… related? Pediatrics. 2021;148(3):e2021052644.
- Lazaros G, Klein AL, Hatziantoniou S, et al. The novel platform of mRNA COVID-19 vaccines and myocarditis: clues into the potential underlying mechanism. Vaccine. 2021;39(35):4925-4927.
- Hasnie AA, Hasnie UA, Patel N, et al. Perimyocarditis following first dose of the mRNA-1273 SARS-CoV-2 (Moderna) vaccine in a healthy young male: a case report. BMC Cardiovasc Disord. 2021;21(1):375.
- McLean K, Johnson TJ. Myopericarditis in a previously healthy adolescent male following COVID-19 vaccination: a case report. Acad Emerg Med. 2021;28(8):918-921.
- Alami A, Krewski D, Farhat N, et al. Risk of myocarditis and pericarditis in mRNA COVID-19-vaccinated and unvaccinated populations: a systematic review and meta-analysis. BMJ Open. 2023;13(6):e065687.
- Yasmin F, Najeeb H, Naeem U, et al. Adverse events following COVID-19 mRNA vaccines: a systematic review of cardiovascular complication, thrombosis, and thrombocytopenia. Immun Inflamm Dis. 2023;11(3):e807.
- Fatima M, Khan MHA, Ali MS, et al. Development of myocarditis and pericarditis after COVID-19 vaccination in children and adolescents: a systematic review. Clin Cardiol. 2023;46(3):243-259.
- Straus W, Urdaneta V, Esposito DB, et al. Analysis of myocarditis among 252 million mRNA-1273 recipients worldwide. Clin Infect Dis. 2023;76(3):e544-e552.
- Naveed Z, Li J, Spencer M, Wilton J, et al. Observed versus expected rates of myocarditis after SARS-CoV-2 vaccination: a population-based cohort study. CMAJ. 2022;194(45):E1529-E1536.
- Corrao G, Franchi M, Cereda D, et al. Increased risk of myocarditis and pericarditis and reduced likelihood of severe clinical outcomes associated with COVID-19 vaccination: a cohort study in Lombardy, Italy. BMC Infect Dis. 2022;22(1):844.
- Chua GT, Kwan MYW, Chui CSL, et al. Epidemiology of acute myocarditis/pericarditis in Hong Kong adolescents following Comirnaty vaccination. Clin Infect Dis. 2022;75(4):673-681.
- Perez Y, Levy ER, Joshi AY, et al. Myocarditis following coronavirus disease 2019 mRNA vaccine: a case series and incidence rate determination. Clin Infect Dis. 2022;75(1):e749-e754.
- Dini FL, Franzoni F, Scarfò G, et al. Acute pericarditis in patients receiving coronavirus disease 2019 vaccines: a case series from the community. J Cardiovasc Med (Hagerstown). 2022;23(8):551-558.
- Sharff KA, Dancoes DM, Longueil JL, et al. Risk of myopericarditis following COVID-19 mRNA vaccination in a large integrated health system: a comparison of completeness and timeliness of two methods. Pharmacoepidemiol Drug Saf. 2022;31(8):921-925.
- Lane S, Yeomans A, Shakir S. Systematic review of spontaneous reports of myocarditis and pericarditis in transplant recipients and immunocompromised patients following COVID-19 mRNA vaccination. BMJ Open. 2022;12(7):e060425.
- Behers BJ, Patrick GA, Jones JM, et al. Myocarditis following COVID-19 vaccination: a systematic review of case reports. Yale J Biol Med. 2022;95(2):237-247.
- Wong HL, Hu M, Zhou CK, et al. Risk of myocarditis and pericarditis after the COVID-19 mRNA vaccination in the USA: a cohort study in claims databases. Lancet. 2022;399(10342):2191-2199.
- Freise NF, Kivel M, Grebe O, et al. Acute cardiac side effects after COVID-19 mRNA vaccination: a case series. Eur J Med Res. 2022;27(1):80.
- Buchan SA, Seo CY, Johnson C, et al. Epidemiology of myocarditis and pericarditis following mRNA vaccination by vaccine product, schedule, and interdose interval among adolescents and adults in Ontario, Canada. JAMA Netw Open. 2022;5(6):e2218505.
- Ahmed SK, Mohamed MG, Essa RA, et al. Global reports of myocarditis following COVID-19 vaccination: a systematic review and meta-analysis. Diabetes Metab Syndr. 2022;16(6):102513.
- Krychtiuk KA, Newby LK. Moderna COVID-19 vaccine was linked to myocarditis or myopericarditis at 28 d (4.2 events/100 000 persons). Ann Intern Med. 2022;175(5):JC58.
- Block JP, Boehmer TK, Forrest CB, et al. Cardiac complications after SARS-CoV-2 infection and mRNA COVID-19 vaccination―PCORnet, United States, January 2021-January 2022. MMWR Morb Mortal Wkly Rep. 2022;71(14):517-523.
- Oster ME, Shay DK, Su JR, et al. Myocarditis cases reported after mRNA-based COVID-19 vaccination in the US from December 2020 to August 2021. JAMA. 2022;327(4):331-340.
- Husby A, Hansen JV, Fosbøl E, et al. SARS-CoV-2 vaccination and myocarditis or myopericarditis: population based cohort study. BMJ. 2021;375:e068665.
- Kim HW, Jenista ER, Wendell DC, et al. Patients with acute myocarditis following mRNA COVID-19 vaccination. JAMA Cardiol. 2021;6(10):1196-1201.
- Nassar M, Nso N, Gonzalez C, et al. COVID-19 vaccine-induced myocarditis: case report with literature review. Diabetes Metab Syndr. 2021;15(5):102205.
- Gargano JW, Wallace M, Hadler SC, et al. Use of mRNA COVID-19 vaccine after reports of myocarditis among vaccine recipients: update from the Advisory Committee on Immunization Practices―United States, June 2021. MMWR Morb Mortal Wkly Rep. 2021;70(27):977-982.
- Faksova K, Walsh D, Jiang Y, et al. COVID-19 vaccines and adverse events of special interest: a multinational Global Vaccine Data Network (GVDN) cohort study of 99 million vaccinated individuals. Vaccine. 2024;42(9):2200-2211.
- Shabu A, Nishtala PS. Analysis of the adverse events following the mRNA-1273 COVID-19 vaccine. Expert Rev Vaccines. 2023;22(1):801-812.
- Kim DH, Kim JH, Oh IS, et al. Adverse events following COVID-19 vaccination in adolescents: insights from pharmacovigilance study of VigiBase. J Korean Med Sci. 2024;39(8):e76.
- Krupickova S, Voges I, Mohiaddin R, et al. Short-term outcome of late gadolinium changes detected on cardiovascular magnetic resonance imaging following coronavirus disease 2019 Pfizer/BioNTech vaccine-related myocarditis in adolescents. Pediatr Radiol. 2023;53(5):892-899.
- Hsu WF, Hsu CH, Jeng MJ. Echocardiographic function evaluation in adolescents following BNT162b2 Pfizer-BioNTech mRNA vaccination: a preliminary prospective study. J Chin Med Assoc. 2024;87(1):88-93.
- Ammirati E, Lupi L, Palazzini M, et al. Outcome and morphofunctional changes on cardiac magnetic resonance in patients with acute myocarditis following mRNA COVID-19 vaccination. Circ Heart Fail. 2023;16(6):e010315.
- Semenzato L, Le Vu S, Botton J, et al. Long-term prognosis of patients with myocarditis attributed to COVID-19 mRNA vaccination, SARS-CoV-2 infection, or conventional etiologies. JAMA. Published online ahead of print August 26, 2024.
- CDC. Clinical considerations: myocarditis and pericarditis after receipt of COVID-19 vaccines among adolescents and young adults. October 10, 2023. https://www.cdc.gov/vaccines/covid-19/clinical-considerations/myocarditis.html. Accessed September 19, 2024.
- Ahsanuddin S, Jin R, Dhanda AK, et al. Otolaryngologic side effects after COVID-19 vaccination. Laryngoscope. 2024;134(3):1163-1168.
- Shoaibi A, Lloyd PC, Wong HL, et al. Evaluation of potential adverse events following COVID-19 mRNA vaccination among adults aged 65 years and older: two self-controlled studies in the U.S. Vaccine. 2023;41(32):4666-4678.
- Shasha D, Bareket R, Sikron FH, et al. Real-world safety data for the Pfizer BNT162b2 SARS-CoV-2 vaccine: historical cohort study. Clin Microbiol Infect. 2022;28(1):130-134.
- van der Boom MDX, van Eekeren R, van Hunsel FPAM. Observed-over-expected analysis as additional method for pharmacovigilance signal detection in large-scaled spontaneous adverse event reporting. Pharmacoepidemiol Drug Saf. 2023;32(7):783-794.
- Rafati A, Pasebani Y, Jameie M, et al. Association of SARS-CoV-2 vaccination or infection with Bell palsy: a systematic review and meta-analysis. JAMA Otolaryngol Head Neck Surg. 2023;149(6):493-504.
- Wan EYF, Chui CSL, Ng VWS, et al. Messenger RNA coronavirus disease 2019 (COVID-19) vaccination with BNT162b2 increased risk of Bell’s palsy: a nested case-control and self-controlled case series study. Clin Infect Dis. 2023;76(3):e291-e298.
- Rzymski P. Guillain-Barré syndrome and COVID-19 vaccines: focus on adenoviral vectors. Front Immunol. 2023;14:1183258.
- Abara WE, Gee J, Marquez P, et al. Reports of Guillain-Barré syndrome after COVID-19 vaccination in the United States. JAMA Netw Open. 2023;6(2):e2253845.
- Bishara H, Arbel A, Barnett-Griness O, et al. Association between Guillain-Barré syndrome and COVID-19 infection and vaccination: a population-based nested case-control study. Neurology. 2023;101(20):e2035-e2042.
- Rüggeberg JU, Gold MS, Bayas JM, et al; Brighton Collaboration Anaphylaxis Working Group. Anaphylaxis: case definition and guidelines for data collection, analysis, and presentation of immunization safety data. Vaccine. 2007;25(31):5675-5684.
- Imai K, Tanaka F, Kawano S, et al. Incidence and risk factors of immediate hypersensitivity reactions and immunization stress-related responses with COVID-19 mRNA vaccine. J Allergy Clin Immunol Pract. 2022;10(10):2667-2676.e10.
- Macy E, Pandya S, Sheikh J, et al. Population-based incidence, severity, and risk factors associated with treated acute-onset COVID-19 mRNA vaccination-associated hypersensitivity reactions. J Allergy Clin Immunol Pract. 2022;10(3):827-836.
- Bousquet J, Agache I, Blain H, et al. Management of anaphylaxis due to COVID-19 vaccines in the elderly. Allergy. 2021;76(10):2952-2964.
- Gaspar A, Moura AL, Cruz C, et al. Polythylene glycol severe allergy and SARS-CoV-2 vaccines: usefulness of testing with PEG 1500 extract. Eur Ann Allergy Clin Immunol. 2023;55(6):261-270.
- Sellaturay P, Nasser S, Islam S, et al. Polyethylene glycol (PEG) is a cause of anaphylaxis to the Pfizer/BioNTech mRNA COVID-19 vaccine. Clin Exp Allergy. 2021;51(6):861-863.
- Troelnikov A, Perkins G, Yuson C, et al. Basophil reactivity to BNT162b2 is mediated by PEGylated lipid nanoparticles in patients with PEG allergy. J Allergy Clin Immunol. 2021;148(1):91-95.
- Paul P, Janjua E, AlSubaie M, et al. Anaphylaxis and related events following COVID-19 vaccination: a systematic review. J Clin Pharmacol. 2022;62(11):1335-1349.
- Zhou ZH, Cortese MM, Fang JL, et al. Evaluation of association of anti-PEG antibodies with anaphylaxis after mRNA COVID-19 vaccination. Vaccine. 2023;41(28):4183-4189.
- Chu DK, Abrams EM, Golden DBK, et al. Risk of second allergic reaction to SARS-CoV-2 vaccines: a systematic review and meta-analysis. JAMA Intern Med. 2022;182(4):376-385.
- CDC. Vaccines & immunizations: interim clinical considerations for use of COVID-19 vaccines in the United States. https://www.cdc.gov/vaccines/covid-19/clinical-considerations/interim-considerations-us.html#history-allergies-reactions. Accessed September 20, 2024.
- Bshesh K, Khan W, Vattoth AL, et al. Lymphadenopathy post-COVID-19 vaccination with increased FDG uptake may be falsely attributed to oncological disorders: a systematic review. J Med Virol. 2022;94(5):1833-1845.
- Tan NJH, Tay KXJ, Wong SBJ, Nga ME. COVID-19 post-vaccination lymphadenopathy: report of cytological findings from fine needle aspiration biopsy. Diagn Cytopathol. 2021;49(12):E467-E470.
- El-Sayed MS, Wechie GN, Low CS, et al. The incidence and duration of COVID-19 vaccine-related reactive lymphadenopathy on 18F-FDG PET-CT. Clin Med (Lond). 2021;21(6):e633-e638.
- Hagen C, Nowack M, Messerli M, et al. Fine needle aspiration in COVID-19 vaccine-associated lymphadenopathy. Swiss Med Wkly. 2021;151:w20557.
- Lim J, Khil EK, Lee SA, et al. Analysis of clinical factors and ultrasound features associated with COVID-19 vaccine-related axillary lymphadenopathy: a large group study. Clin Imaging. 2024;105:110046.
- Yoshikawa T, Miki S, Nakao T, et al. Axillary lymphadenopathy after Pfizer-BioNTech and Moderna COVID-19 vaccination: MRI evaluation. Radiology. 2023;306(1):270-278.
- Mema E, Lane EG, Drotman MB, et al. Axillary lymphadenopathy after a COVID-19 vaccine booster dose: time to resolution on ultrasound follow-up and associated factors. AJR Am J Roentgenol. 2023;221(2):175-183.
- Lane EG, Eisen CS, Drotman MB, et al. Time for resolution of COVID-19 vaccine-related axillary lymphadenopathy and associated factors. AJR Am J Roentgenol. 2022;219(4):559-568.
- Zhang M, Ahn RW, Hayes JC, et al. Axillary lymphadenopathy in the COVID-19 era: what the radiologist needs to know. Radiographics. 2022;42(7):1897-1911.
- Twentyman E, Wallace M, Roper LE, et al. Interim recommendation of the Advisory Committee on Immunization Practices for use of the Novavax COVID-19 vaccine in persons aged ≥18 years — United States, July 2022. MMWR Morb Mortal Wkly Rep. 2022;71:988-992.
- Keech C, Albert G, Cho I, et al. Phase 1-2 trial of a SARS-CoV-2 recombinant spike protein nanoparticle vaccine. N Engl J Med. 2020;383(24):2320-2332.
- Shinde V, Bhikha S, Hoosain Z, et al; 2019nCoV-501 Study Group. Efficacy of NVX-CoV2373 Covid-19 vaccine against the B.1.351 variant. N Engl J Med. 2021;384(20):1899-1909.
- Heath PT, Galiza EP, Baxter DN, et al; 2019nCoV-302 Study Group. Safety and efficacy of NVX-CoV2373 Covid-19 vaccine. N Engl J Med. 2021;385(13):1172-1183.
- Stuart ASV, Shaw RH, Liu X, et al; Com-COV2 Study Group. Immunogenicity, safety, and reactogenicity of heterologous COVID-19 primary vaccination incorporating mRNA, viral-vector, and protein-adjuvant vaccines in the UK (Com-COV2): a single-blind, randomised, phase 2, non-inferiority trial. Lancet. 2022;399(10319):36-49.
- Dunkle LM, Kotloff KL, Gay CL, et al; 2019nCoV-301 Study Group. Efficacy and safety of NVX-CoV2373 in adults in the United States and Mexico. N Engl J Med. 2022;386(6):531-543.
- Heath PT, Galiza EP, Baxter DN, et al. Safety and efficacy of the NVX-CoV2373 coronavirus disease 2019 vaccine at completion of the placebo-controlled phase of a randomized controlled trial. Clin Infect Dis. 2023;76(3):398-407.
- Madhi SA, Moodley D, Hanley S, et al; 2019nCoV-501 Study Group. Immunogenicity and safety of a SARS-CoV-2 recombinant spike protein nanoparticle vaccine in people living with and without HIV-1 infection: a randomised, controlled, phase 2A/2B trial. Lancet HIV. 2022;9(5):e309-e322.
- Underwood E, Dunkle LM, Madhi SA, et al. Safety, efficacy, and immunogenicity of the NVX-CoV2373 vaccine. Expert Rev Vaccines. 2023;22(1):501-517.
- Marchese AM, Kalkeri R, Vadivale M, et al. Pivoting to protein: the immunogenicity and safety of protein-based NVX-CoV2373 as a heterologous booster for inactivated and viral vector COVID-19 vaccines. Expert Rev Vaccines. 2023;22(1):620-628.
- Kim S, Ko M, Heo Y, et al. Safety surveillance of the NVX-CoV2373 COVID-19 vaccine among Koreans aged 18 years and over. Vaccine. 2023;41(35):5066-5071.
- Raiser F, Davis M, Adelglass J, et al; 2019nCoV-307 Study Team. Immunogenicity and safety of NVX-CoV2373 as a booster: a phase 3 randomized clinical trial in adults. Vaccine. 2023;41(41):5965-5973.
- Sheng WH, Lin PH, Cheng YC, et al. Immunogenicity and safety of heterologous booster with protein-based COVID-19 vaccine (NVX-CoV2373) in healthy adults: a comparative analysis with mRNA vaccines. J Formos Med Assoc. 2024;123(3):340-346.
- Mallory RM, Formica N, Pfeiffer S, et al; Novavax 2019nCoV101 Study Group. Safety and immunogenicity following a homologous booster dose of a SARS-CoV-2 recombinant spike protein vaccine (NVX-CoV2373): a secondary analysis of a randomised, placebo-controlled, phase 2 trial. Lancet Infect Dis. 2022;22(11):1565-1576.
- Kim HY, Cho JY, Yoon HJ, et al. A case report for acute myopericarditis after NVX-CoV2373 (Novavax®) COVID-19 vaccination. J Korean Med Sci. 2022;37(34):e265.
- Fix J, Christopher Mast T, Smith K, Baker N. Benefit-risk assessment for the Novavax COVID-19 vaccine (NVX-CoV2373). Vaccine. 2024;42(9):2161-2165.
- Vaccine Error Reporting System. VAERS home: report an adverse drug event to VAERS. https://vaers.hhs.gov/reportevent.html. Accessed September 20, 2024.
- American Society of Health System Pharmacists Council on Professional Affairs. ASHP guidelines on the pharmacist’s role in immunization. Am J Health Syst Pharm. 2003;60(13):1371-1377.
The content contained in this article is for informational purposes only. The content is not intended to be a substitute for professional advice. Reliance on any information provided in this article is solely at your own risk.
To comment on this article, contact rdavidson@uspharmacist.com.






