US Pharm. 2024;49(5):22-34.
ABSTRACT: Bipolar disorder (BD) is a complex and lifelong mental health disorder characterized by episodes of mania, hypomania, and/or depression. Affected individuals are often diagnosed with comorbidities including metabolic syndrome and substance use disorder, and life expectancy is partly reduced by suicidal ideation. Current pharmacotherapies, many of which were serendipitously discovered, sufficiently target aberrant neurotransmission but yield inadequate outcomes and cause burdensome side effects. Alternative treatments with fewer adverse effects are in development. Some alternative pharmacotherapies entail reappropriation of classic drugs, such as ketamine and scopolamine. Nonpharmacologic treatments help individuals develop coping mechanisms, improve medication adherence, and establish mood-stabilizing social rhythms.
Bipolar disorder (BD) is a severe and chronic mental health disorder characterized by intense mood swings that are often irrespective of external circumstances.1 Mood ranges from euphoria (highs) to depression (lows).1 Despite its classification as a “mood disorder,” BD also negatively impacts cognition and behavior.2 Individuals with BD can experience feelings of guilt and worthlessness, engage in substance abuse, and consider suicide, with 25% to 60% of affected individuals attempting suicide and 5% to 20% following through with it.2-6 Suicidal behavior is more strongly associated with mixed states (i.e., simultaneous symptoms of mania and depression) than with pure mania or hypomania, largely attributable to increased time spent in a depressive state.2,7,8 Psychotic events, including delusions and hallucinations (more associated with bipolar mania), are possible.9 Individuals with BD commonly exhibit comorbidities, including cardiovascular and metabolic diseases (e.g., hypertension, dyslipidemia, hypertriglyceridemia, insulin resistance, abdominal obesity), which can further impair executive function.10-12 Life expectancy is generally reduced by at least a decade.13 According to the Depression and Bipolar Support Alliance, a nonprofit organization providing support for people with BD and depression, approximately 5.7 million Americans aged ≥18 years (~2.6% of the population) have been diagnosed with BD.14 The current National Institute of Mental Health estimate is slightly higher at 2.8%.15 Epidemiologic data indicate that BD is equally distributed across sex, ethnicity, and geographic area (urban vs. rural).16,17
The American Psychiatric Association’s Diagnostic and Statistical Manual of Mental Disorders (DSM-5) delineates BD subtypes, including bipolar I (BD I), bipolar II (BD II), and cyclothymia (see TABLE 1).18 A manic episode, or mania, is described as a distinct period of abnormally and persistently elevated mood that lasts for at least 1 week (or shorter if hospitalization is required) and is associated with significant impairment, psychosis, and/or need for hospitalization.5,19 Manic symptoms include abnormally elevated mood (i.e., euphoria, irritability, hyperactivity).2,18 A hypomanic mood episode resembles mania; however, it has a shorter duration, lasting only 4 days.2,5,18 Hypomania is not severe enough to cause marked impairment or require hospitalization.2 Depressive symptomology includes depressed mood and an emotional state manifesting as anhedonia, apathy, sadness, hopelessness, and pessimism.5 Thus, a distinguishing feature between BD I and BD II is the presence of either a manic or hypomanic state, respectively.20 Multiple grading scales are used to categorize mood disorders and select appropriate treatment regimens, including the Montgomery-Åsberg Depression Rating Scale (MADRS) and Young Mania Rating Scale (YMRS).21 The YMRS quantitatively assesses manic symptom severity, whereas the MADRS monitors changes in depressive symptom severity in response to antidepressant therapy.22,23 The U.S. aggregate lifetime (and 12-month) prevalence estimates are 1.0% (0.6%) for BD I and 1.1% (0.8%) for BD II.24

Etiology and Pathophysiology
The etiopathophysiology of BD remains largely unknown. It likely involves a complex interplay of genetic, epigenetic, neurochemical, and environmental factors.25 Imbalances in key intracellular signaling systems that regulate mood and behavior are implicated. Upward of 30 dysregulated genes are linked to an increased risk of developing BD.25,26 These genes encode for various neurotransmitter receptors (e.g., dopamine, serotonin) as well as regulatory enzymes involved in neurotransmitter production, breakdown, and cellular detoxification (e.g., aldehyde dehydrogenase, alcohol dehydrogenase, monoamine oxidase, tryptophan hydroxylase).19,25 Normal levels of neurotrophins, such as brain-derived neurotrophic factor (BDNF), nerve growth factor, neurotrophin-3, and neurotrophin-4, are important for mediating neuroplasticity (i.e., the ability of neural networks in the brain to adapt, grow, and reorganize via structural and functional changes).27,28 A reduced BDNF serum level, a protein that plays key roles in neuronal survival and growth, neurotransmitter modulation, and neuronal plasticity critical for learning and memory, is implicated in the development of BD.29-33 Regarding genetic susceptibility, children of one parent or both parents with a history of BD have a 15% to 30% and 50% to 75% risk of developing the disorder themselves, respectively.14 Other culprits include mitochondrial dysfunction, oxidative stress, immune-inflammatory imbalance, and a compromised hypothalamic-pituitary-adrenal axis.25 Childhood mistreatment (emotional abuse, neglect, trauma) is also linked to an increased risk of developing BD.25 Nearly 60% of adults report a stressful trigger before a manic or depressive episode.17,25 Additional risk factors include prenatal and perinatal factors (i.e., perinatal infections, obstetric complications), psychological stressors (i.e., life events), substance misuse (e.g., cannabis, opioids, tranquilizers, stimulants, sedatives, cocaine), and medical comorbidities (e.g., irritable bowel syndrome, asthma, obesity, migraines, multiple sclerosis).17
Diagnosis
BD I: An individual must experience at least one manic episode that is unattributable to schizoaffective disorder, schizophrenia, schizophreniform disorder, delusional disorder, or any other psychotic disorder.18,34 When selecting a diagnostic code, the following items should be listed sequentially: BD I, type of episode experienced including severity (mild, moderate, or severe); psychotic features; and remission status.18,34 The diagnostic code should be followed by as many uncoded specifiers as apply to the current or most recent episode.18,34
BD II: An individual must experience a current hypomanic episode or have experienced one previously.18,34 At least one major depressive episode that is not attributable to any other mood disorder must also be experienced.18,34 BD II patients experience anguish and limitations to functional ability (e.g., social, occupational) due to the depression experienced.18,34 Although the type of current or most recent episode as well as severity/psychotic/remission specifiers are not accounted for in a BD II diagnostic code, these characteristics should still be specified in writing following one specific diagnostic code (ICD-10 F31.81).18,34
Current Pharmacotherapies
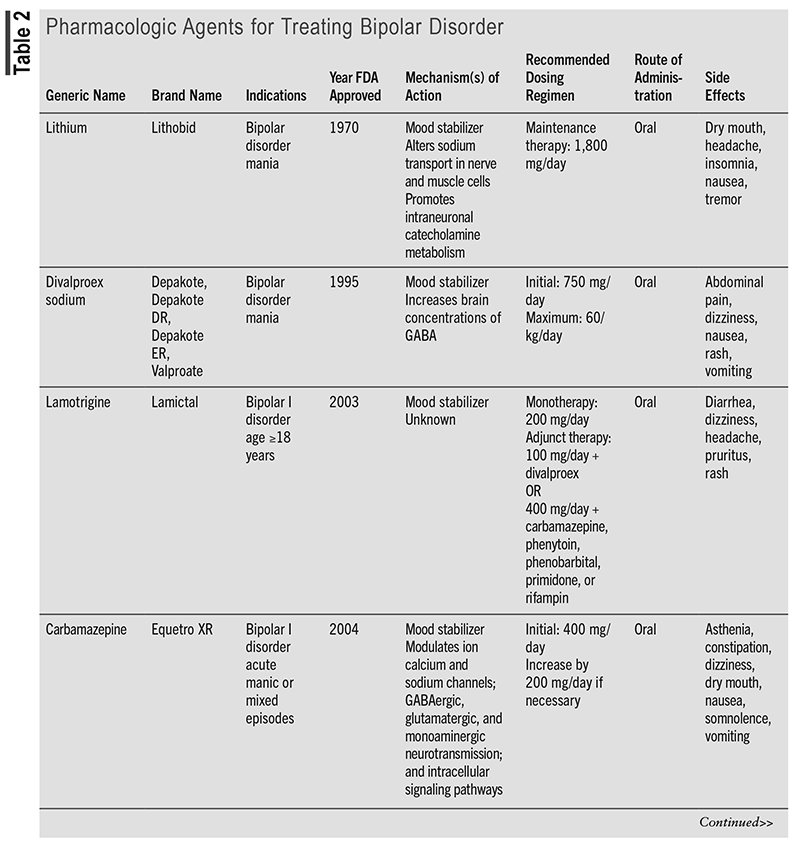
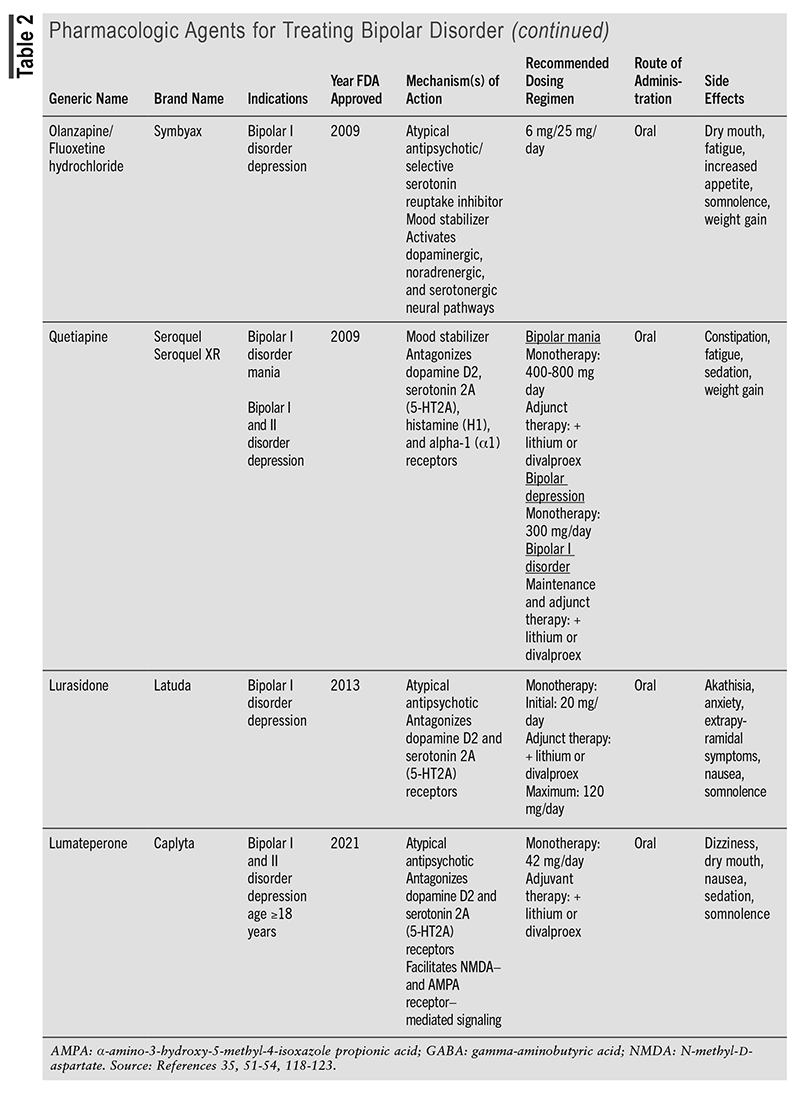
Mainstay BD medications are mood stabilizers, atypical antipsychotics, and antidepressants (TABLE 2). Although their mechanisms of action are generally unclear, they restore normal adrenergic, catecholaminergic, dopaminergic, gamma-aminobutyric acid-ergic (GABAergic), glutamatergic, histaminergic, ionic, and serotonergic signaling. Safety and efficacy require agent- and BD subtype–specific dosing regimens. Despite often being serendipitously discovered and sometimes achieving suboptimal outcomes, they display varying degrees of superiority over placebo and/or alternative BD medications as monotherapy or adjunctive therapy in terms of alleviating symptom severity (as assessed by BD grading scales) and prolonging time to escalated intervention (e.g., hospitalization).35-49 Alarmingly, long-term use of lithium, the first-line and cornerstone maintenance therapy for bipolar mania, is linked to decreased kidney concentrating ability, hypothyroidism, hypercalcemia, and hyperparathyroidism.50 Lamotrigine (Lamictal) is a BD I maintenance treatment that is administered in combination with lithium in adults experiencing acute BD mood episodes because it delays time to occurrence.47,51,52 Lumateperone (Caplyta), a more recently FDA-approved medication for BD I and BD II depression, differs from other agents by its high affinity and association for the 5-HT2A receptor, with less D2 and alpha-1 adrenergic receptor affinity.53,54 This feature results in fewer side effects (little to no weight gain, fewer extrapyramidal symptoms [EPS]). Lumateperone has no known contraindications other than hypersensitivity to drug ingredients; however, patients should avoid concomitant use with cytochrome P450 3A4 (CYP3A4) inhibitors or inducers and if they have moderate-to-severe hepatic impairment.55
Emerging Treatments
Despite their benefits, currently used medications can elicit bothersome and potentially serious side effects that might hinder patient compliance and cause significant harm. Pharmacologic treatments as well as nonpharmacologic therapies with fewer adverse effects and more tolerability are in development. They generally work to ameliorate disease-causing neurotransmitter dysfunction, perturbed cerebral glucose metabolism, and oxidative stress.
Ketamine: This anesthetic, antidepressant, and psychotomimetic agent is an N-methyl-D-aspartate (NMDA) receptor antagonist.56 Utilized for treatment-resistant, unipolar major depression and suicide ideation, low-dose IV ketamine infusion as standalone treatment at subpsychedelic dosages is also generally safe and effective for treatment-resistant BD.57-64 Antidepressant actions target lack of interest and enjoyment.65 Adverse effects include dissociation, elevated pulse and/or blood pressure, headache, nausea, anxiety, changes in vision, and muscle tension; however, these effects are generally well tolerated and usually abate within 2 hours of administration.59,66 Typically dosed at 0.5 mg/kg, IV ketamine has a rapid onset (~30 seconds) and short elimination half-life (~2-4 hours), but its antidepressant effect lasts for 1 week following a single infusion.63 The treatment holds superiority over placebo for up to 12 days, with 1-week-long superiority consistently observed in BD patients.57 BD patients display alterations in glutamatergic dysfunction and cerebral glucose metabolism in brain regions involved in emotion and cognition, including the amygdala-sensorimotor pathway, hippocampus, and prefrontal cortex.67,68 In preclinical mouse studies, ketamine improved depressive-like behavior by increasing cerebral glucose uptake; in humans, it facilitates prefrontal cortex glutamatergic neurotransmission and restores normal glucose metabolism.69-71 Although several current clinical trials report promising results with acute use, more are needed to further ascertain the tolerability, safety, and efficacy of long-term use. Further studies are also required to address potential withdrawal effects since ketamine acts at the mu-opioid receptor.72 Frequent IV infusions of racemic ketamine may be intolerable, so ongoing studies are evaluating the efficacy of esketamine, the L-isomer of ketamine, nasal spray (Spravato).73
Scopolamine: This agent is utilized to manage postoperative nausea, motion sickness, and hypersalivation.74 Due to its antidepressant properties, it is being investigated as a rapid treatment option to reduce depressive symptoms in moderate-to-severe BD.75 The antidepressant actions of scopolamine were gleaned from observations that physostigmine, a cholinesterase inhibitor, exacerbated depressive symptoms and promoted emotional instability in individuals with BD.76 Scopolamine exerts opposite effects to those of physostigmine by inhibiting postganglionic cholinergic receptors. As a pan-muscarinic antagonist, scopolamine reduces the hypersensitivity associated with depressive episodes.77 Findings from studies examining the utility of IV scopolamine in individuals with schizophrenia are promising and indicate potential for overlapping use in BD with no serious adverse effects.78 A phase IIb clinical trial investigating the use of IV scopolamine to treat BD is currently under way.79 Successful outcomes with IV scopolamine could pave the way for at-home administration as a dermal patch.80
Dexmedetomidine: This agent (Igalmi) is a selective alpha-2 adrenergic receptor agonist with a quick onset of action and short half-life.81 Its hypnotic action has been leveraged to successfully treat mild-to-moderate agitation associated with BD and schizophrenia.81-83 The noninvasive sublingual formulation (120-mg and 180-mg doses) exhibits no dopaminergic activity, which prevents EPS, such as akathisia and dystonia; these adverse effects are commonly seen with other treatments for acute agitation (e.g., intramuscular benzodiazepines).81,84 Patients aged ≥65 years or with any degree of hepatic impairment should be dose-adjusted accordingly and may require subsequent dosing after initial administration.85 Concomitant use with opioids, hypnotics, sedatives, anesthetics, and medications that may cause QT prolongation should be avoided, as dexmedetomidine is metabolized by cytochrome P450 2A6 (CYP2A6).85
Risperidone: FDA-approved in 2023 and branded as Rykindo, this serotonin and norepinephrine reuptake inhibitor is a once-biweekly IM gluteal injection used to treat schizophrenia in adult patients.86 It is currently being investigated as a potential BD treatment. Injection applies microsphere technology to deliver a long-acting, extended-release formulation of risperidone as an adjunctive therapy to lithium or divalproex, which could improve adherence and disorder management.87,88 Individuals must establish oral tolerability to risperidone, as the injection must be initially administered with 7 days of oral risperidone. The recommended IM dosage is 25 mg every 2 weeks, with titration up to 37.5 mg or 50 mg at intervals of at least 4 weeks.89 There are many warnings for risperidone, the most concerning being that it can cause death in elderly patients with dementia-related psychosis due to increased risk for adverse cardiovascular events, such as transient ischemic attacks and stroke.89 Additional side effects include significant weight gain, tremor, and Parkinsonism.89,90 Patients should be titrated appropriately if they have renal or hepatic impairment and should avoid use with strong CYP2D6 inhibitors and strong CYP3A4 inducers.87
Cognitive Behavioral Therapy (CBT): CBT operates on the premise that thoughts, mood, and behavior influence each other and guides individuals to acknowledge their diagnosis, overcome depressive episodes, reconstruct negative thoughts, and recognize prodromal symptoms to prevent relapse.34,101 In BD I patients, addition of CBT to mood-stabilizer pharmacotherapy results in higher social functioning, fewer mood symptoms, better coping mechanisms, and reduced fluctuations in manic symptoms compared with patients who are treated with mood-stabilizing agents only.102 Regular engagement in therapy sessions may help BD patients develop a form of cognitive control over their condition and prevent relapse.
Interpersonal and Social Rhythm Therapy (IPSRT): IPSRT focuses on averting disease flares and/or prolonging the intervals between occurrences.103 IPSRT achieves this in BD patients by improving medication adherence, resolving stressful interpersonal situations, and reducing disruptions in social rhythms (e.g., patterns of daily routines and interactions).103 Patients diagnosed with BD I who receive IPSRT live longer without a relapse and display more consistent social rhythms.104
Transcranial Magnetic Stimulation (TMS): TMS is a noninvasive, brain-stimulating therapy for treatment-resistant depression approved by the FDA in 2008.105 TMS administers a swift current pulse through a coil positioned over the scalp to stimulate cortical activity and evoke neuronal action potentials.106 TMS may be effective for treatment-resistant BD depressive episodes, as ~50% of treatment-resistant BD participants respond favorably to TMS treatment, with an additional ~25% partially responding.105
Investigational Approach
Calcium-Calmodulin–Dependent Protein Kinase Kinase-2 Activators (CaMKK2): The neuronal enzyme CaMKK2 regulates bioenergetic, metabolic, and neuronal processes governing higher order cognitive and behavioral functions, including mood, long-term memory, and other affective functions.91 CaMKK2 loss-of-function genetic polymorphisms and missense mutations are linked to disease etiopathophysiology.91 In preclinical mouse studies, genetic deletion of CaMKK2 results in bipolar-like behaviors that are ameliorated by lithium, which increases CaMKK2 activity.92 Oxidative stress, a hallmark of BD, increases with disorder severity.93 Reactive oxygen species partly derived from increased lipid peroxidation rise with CaMKK2 deficiency and are elevated in serum from BD patients.94-98 CaMKK2 suppresses lipid peroxidation by increasing the activity of a transcription factor that promotes the expression of antioxidant and detoxification enzymes, as well as that of the glutathione and thioredoxin antioxidant systems.99,100 Activating CaMKK2-mediated signaling, therefore, shows potential as a viable pharmacotherapeutic strategy.
Challenges With Emerging Therapies
Novel therapies do have drawbacks. Drowsiness is possible with ketamine, so individuals should refrain from possibly unsafe activities within 24 hours of undergoing treatment.107 Scopolamine use carries seizure potential.108 With dexmedetomidine, patients should report any experiences of weakness, confusion, or excessive sweating within 48 hours of treatment.109 Risperidone should be cautiously used during pregnancy and avoided with breastfeeding over concerns about it affecting newborns postnatally and passing into breastmilk.110 CBT can make individuals emotionally uncomfortable because it involves exploring potentially painful feelings and experiences.111 Side effects of TMS include scalp discomfort and pain, headache, lightheadedness, and tingling, spasms, or twitching of facial muscles; however, these symptoms are typically mild to moderate, improve shortly after a session, and diminish with repeat sessions.112 Taken together, individual-specific tolerability should be prioritized.
The Pharmacist’s Role
According to the 2012 National Alliance on Mental Illness national survey, only 53% of individuals who are prescribed mental health medications reported a strong relationship with their pharmacist, with 43% reporting no relationship.113 Disappointingly, 75% of respondents reported not receiving effective pharmacist-led assistance, including safety monitoring.113 Medication nonadherence is a major treatment barrier, with ~50% of BD patients nonadherent or partially adherent.114 Common adverse effects of mainstay pharmacotherapies are weight gain, metabolic dysregulation, sedation/somnolence, and cardiovascular issues (e.g., hypertension, myocardial infarction, stroke).115 Other contributing factors are complex dosing regimens, skepticism about poorly understood medications, rapid cycling BD, substance use, and weak therapeutic alliance.116 Pharmacists should regularly discuss therapeutic versus adverse medication effects to promote adherence.117 Counseling points include nonpharmacologic strategies to mitigate weight gain, such as lifestyle modification (i.e., exercise, dietary changes, cognitive interventions), as well as pharmacologic treatments to combat overweight/obesity, Parkinsonism, insulin resistance, hyperglycemia, sedation, gastroesophageal reflux disease, and seizure activity.117
Conclusion
Individuals with BD face significant disease burdens, and conventional treatments do not always achieve optimal outcomes. Emerging pharmacologic and nonpharmacologic treatment approaches that are more targeted show promise in terms of improved efficacy and tolerability and appear to be generally safe.
REFERENCES
1. Gooding D. Bipolar Disorder.; 2023. https://research-ebsco-com.wmcarey.idm.oclc.org/c/57i3iz/viewer/html/nqdbdwa5z5?route=details.
2. Keck PEJ, McElroy SL, Arnold LM. Bipolar disorder. Med Clin North Am. 2001;85(3):645-661.
3. Strakowski SM, McElroy SL, Keck PEJ, West SA. Suicidality among patients with mixed and manic bipolar disorder. Am J Psychiatry. 1996;153(5):674-676.
4. Dilsaver SC, Chen YW, Swann AC, et al. Suicidality in patients with pure and depressive mania. Am J Psychiatry. 1994;151(9):1312-1315.
5. Marano G, Traversi G, Nannarelli C, et al. Bipolar Disorder: Symptoms, Management and Risk Factors, Moore NB, ed. Happauge, NY: Nova Science Publishers; 2013.
6. Sublette ME, Carballo JJ, Moreno C, et al. Substance use disorders and suicide attempts in bipolar subtypes. J Psychiatr Res. 2009;43(3):230-238.
7. Persons JE, Coryell WH, Solomon DA, et al. Mixed state and suicide: is the effect of mixed state on suicidal behavior more than the sum of its parts? Bipolar Disord. 2018;20(1):35-41.
8. Kamali M, Reilly-Harrington NA, Chang WC, et al. Bipolar depression and suicidal ideation: moderators and mediators of a complex relationship. J Affect Disord. 2019;259:164-172.
9. Chakrabarti S, Singh N. Psychotic symptoms in bipolar disorder and their impact on the illness: a systematic review. World J Psychiatry. 2022;12(9):1204-1232.
10. Westman J, Hällgren J, Wahlbeck K, et al. Cardiovascular mortality in bipolar disorder: a population-based cohort study in Sweden. BMJ Open. 2013;3(4).
11. Fagiolini A, Chengappa KNR, Soreca I, Chang J. Bipolar disorder and the metabolic syndrome: causal factors, psychiatric outcomes and economic burden. CNS Drugs. 2008;22(8):655-669.
12. Yates KF, Sweat V, Yau PL, et al. Impact of metabolic syndrome on cognition and brain: a selected review of the literature. Arterioscler Thromb Vasc Biol. 2012;32(9):2060-2067.
13. Kessing LV, Vradi E, Andersen PK. Life expectancy in bipolar disorder. Bipolar Disord. 2015;17(5):543-548.
14. Depression and Bipolar Support Alliance. Bipolar Disorder Statistics. www.dbsalliance.org/education/bipolar-disorder/bipolar-disorder-statistics. Accessed February 13, 2024.
15. National Institute of Mental Health (NIMH). Bipolar Disorder. www.nimh.nih.gov/health/statistics/bipolar-disorder. Accessed February 19, 2024.
16. Kroon JS, Wohlfarth TD, Dieleman J, et al. Incidence rates and risk factors of bipolar disorder in the general population: a population-based cohort study. Bipolar Disord. 2013;15(3):306-313.
17. Rowland TA, Marwaha S. Epidemiology and risk factors for bipolar disorder. Ther Adv Psychopharmacol. 2018;8(9):251-269.
18. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. Fifth ed (DSM-5). Arlington, VA: American Psychiatric Association; 2013.
19. Wedig M, Weinstock L. Bipolar Disorder: Causes, Diagnosis and Treatment (Psychology of Emotions, Motivations and Actions: Psychiatry-Theory, Applications and Treatments). Plunkett JM, ed. Happauge, NY: Nova Science Publishers; 2011.
20. Baek JH, Park DY, Choi J, et al. Differences between bipolar I and bipolar II disorders in clinical features, comorbidity, and family history. J Affect Disord. 2011;131(1-3):59-67.
21. Rating scales and safety measurement
22. Quilty LC, Robinson JJ, Rolland JP, et al. The structure of the Montgomery-Åsberg depression rating scale over the course of treatment for depression. Int J Methods Psychiatr Res. 2013;22(3):175-184.
23. Lukasiewicz M, Gerard S, Besnard A, et al. Young Mania Rating Scale: How to interpret the numbers? Determination of a severity threshold and of the minimal clinically significant difference in the EMBLEM cohort. Int J Methods Psychiatr Res. 2013;22(1):46-58.
24. Merikangas KR, Akiskal HS, Angst J, et al. Lifetime and 12-month prevalence of bipolar spectrum disorder in the National Comorbidity Survey replication. Arch Gen Psychiatry. 2007;64(5):543-552.
25. Bipolar Disorder. StatPearls–NCBI Bookshelf. www.ncbi.nlm.nih.gov/books/NBK558998. Accessed February 13, 2024.
26. Kerner B. Genetics of bipolar disorder. Appl Clin Genet. 2014;7:33-42.
27. Puderbaugh M, Emmady P. Neuroplasticity. StatPearls; 2023. www.ncbi.nlm.nih.gov/books/NBK557811/. Accessed February 13, 2024.
28. Habtemariam S. The brain-derived neurotrophic factor in neuronal plasticity and neuroregeneration: new pharmacological concepts for old and new drugs. Neural Regen Res. 2018;13(6):983-984.
29. Scaini G, Valvassori SS, Diaz AP, et al. Neurobiology of bipolar disorders: a review of genetic components, signaling pathways, biochemical changes, and neuroimaging findings. Braz J Psychiatry. 2020;42(5):536-551.
30. Konopaske GT, Lange N, Coyle JT, Benes FM. Prefrontal cortical dendritic spine pathology in schizophrenia and bipolar disorder. JAMA Psychiatry. 2014;71(12):1323-1331.
31. Jain A, Mitra P. Bipolar Disorder. StatPearls; 2023. www.ncbi.nlm.nih.gov/books/NBK558998/. Accessed February 19, 2024.
32. Tunçel ÖK, Sarisoy G, Çetin E. Neurotrophic factors in bipolar disorders patients with manic episode. Turk J Med Sci. 2020;50(4):985-993.
33. Shkundin A, Halaris A. Associations of BDNF/BDNF-AS SNPs with depression, schizophrenia, and bipolar disorder. J Pers Med. 2023;13(9).
34. Black D, Grant J. DSM-5 Guidebook–The Essential Companion to the Diagnostic and Statistical Manual of Mental Disorders, Fifth ed; 2014.
35. Seroquel (quetiapine). Prescribing information. www.fda.gov/medwatch. Accessed February 13, 2024.
36. Sachs G, Chengappa KNR, Suppes T, et al. Quetiapine with lithium or divalproex for the treatment of bipolar mania: a randomized, double-blind, placebo-controlled study. Bipolar Disord. 2004;6(3):213-223.
37. Calabrese JR, Keck PEJ, Macfadden W, et al. A randomized, double-blind, placebo-controlled trial of quetiapine in the treatment of bipolar I or II depression. Am J Psychiatry. 2005;162(7):1351-1360.
38. Bowden CL, Calabrese JR, Sachs G, et al. A placebo-controlled 18-month trial of lamotrigine and lithium maintenance treatment in recently manic or hypomanic patients with bipolar I disorder. Arch Gen Psychiatry. 2003;60(4):392-400.
39. Bowden CL, Swann AC, Calabrese JR, et al. A randomized, placebo-controlled, multicenter study of divalproex sodium extended release in the treatment of acute mania. J Clin Psychiatry. 2006;67(10):1501-1510.
40. Loebel A, Cucchiaro J, Silva R, et al. Lurasidone monotherapy in the treatment of bipolar I depression: a randomized, double-blind, placebo-controlled study. Am J Psychiatry. 2014;171(2):160-168.
41. Loebel A, Cucchiaro J, Silva R, et al. Lurasidone as adjunctive therapy with lithium or valproate for the treatment of bipolar I depression: A randomized, double-blind, placebo-controlled study. Am J Psychiatry. 2014;171(2):169-177.
42. Brown EB, McElroy SL, Keck PEJ, et al. A 7-week, randomized, double-blind trial of olanzapine/fluoxetine combination versus lamotrigine in the treatment of bipolar I depression. J Clin Psychiatry. 2006;67(7):1025-1033.
43. Calabrese JR, Durgam S, Satlin A, et al. Efficacy and safety of lumateperone for major depressive episodes associated with bipolar I or bipolar II disorder: a phase 3 randomized placebo-controlled trial. Am J Psychiatry. 2021;178(12):1098-1106.
44. Weisler RH, Keck PEJ, Swann AC. Extended-release carbamazepine capsules as monotherapy for acute mania in bipolar disorder: a multicenter, randomized, double-blind, placebo-controlled trial. J Clin Psychiatry. 2005;66(3):323-330.
45. Weisler RH. Carbamazepine extended-release capsules in bipolar disorder. Neuropsychiatr Dis Treat. 2006;2(1):3-11.
46. Bowden CL, Grunze H, Mullen J, et al. A randomized, double-blind, placebo-controlled efficacy and safety study of quetiapine or lithium as monotherapy for mania in bipolar disorder. J Clin Psychiatry. 2005;66(1):111-121.
47. Zhihan G, Fengli S, Wangqiang L, et al. Lamotrigine and lithium combination for treatment of rapid cycling bipolar disorder: results from meta-analysis. Front Psychiatry. 2022;13:913051.
48. Geddes JR, Calabrese JR, Goodwin GM. Lamotrigine for treatment of bipolar depression: independent meta-analysis and meta-regression of individual patient data from five randomised trials. Br J Psychiatry. 2009;194(1):4-9.
49. Besag FMC, Vasey MJ, Sharma AN, Lam ICH. Efficacy and safety of lamotrigine in the treatment of bipolar disorder across the lifespan: a systematic review. Ther Adv Psychopharmacol. 2021;11:20451253211045870.
50. Volkmann C, Bschor T, Köhler S. Lithium treatment over the lifespan in bipolar disorders. Front Psychiatry. 2020;11:377.
51. Lamictal (lamotrigine). Prescribing information. www.fda.gov/medwatch. Accessed February 13, 2024.
52. Butler M, Urosevic S, Desai P, et al. Treatment for Bipolar Disorder in Adults: A Systematic Review. Rockville, MD: Agency for Healthcare Research and Quality. 2018.
53. Snyder GL, Vanover KE, Zhu H, et al. Functional profile of a novel modulator of serotonin, dopamine, and glutamate neurotransmission. Psychopharmacology (Berl). 2015;232(3):605-621.
54. Caplyta (lumateperone). Prescribing information. www.fda.gov/medwatch. Accessed February 14, 2024.
55. Meyer. Out of the pipeline [Online]. 2022.
56. Cohen SP, Bhatia A, Buvanendran A, et al. Consensus guidelines on the use of intravenous ketamine infusions for chronic pain from the American Society of Regional Anesthesia and Pain Medicine, the American Academy of Pain Medicine, and the American Society of Anesthesiologists. Reg Anesth Pain Med. 2018;43(5):521-546.
57. Zarate CAJ, Brutsche NE, Ibrahim L, et al. Replication of ketamine’s antidepressant efficacy in bipolar depression: a randomized controlled add-on trial. Biol Psychiatry. 2012;71(11):939-946.
58. Wilkowska A, Włodarczyk A, Gałuszko-Węgielnik M, et al. Intravenous ketamine infusions in treatment-resistant bipolar depression: an open-label naturalistic observational study. Neuropsychiatr Dis Treat. 2021;17:2637-2646.
59. Zarate CAJ, Singh JB, Carlson PJ, et al. A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Arch Gen Psychiatry. 2006;63(8):856-864.
60. Murrough JW, Iosifescu D V, Chang LC, et al. Antidepressant efficacy of ketamine in treatment-resistant major depression: a two-site randomized controlled trial. Am J Psychiatry. 2013;170(10):1134-1142.
61. Diazgranados N, Ibrahim L, Brutsche NE, et al. A randomized add-on trial of an N-methyl-D-aspartate antagonist in treatment-resistant bipolar depression. Arch Gen Psychiatry. 2010;67(8):793-802.
62. Coyle CM, Laws KR. The use of ketamine as an antidepressant: a systematic review and meta-analysis. Hum Psychopharmacol. 2015;30(3):152-163.
63. Matveychuk D, Thomas RK, Swainson J, et al. Ketamine as an antidepressant: overview of its mechanisms of action and potential predictive biomarkers. Ther Adv Psychopharmacol. 2020;10:2045125320916657.
64. Wilkinson ST, Ballard ED, Bloch MH, et al. The Effect of a single dose of intravenous ketamine on suicidal ideation: a systematic review and individual participant data meta-analysis. Am J Psychiatry. 2018;175(2):150-158.
65. Berman RM, Cappiello A, Anand A, et al. Antidepressant effects of ketamine in depressed patients. Biol Psychiatry. 2000;47(4):351-354.
66. Bennett R, Yavorsky C, Bravo G. Ketamine for bipolar depression: Biochemical, psychotherapeutic, and psychedelic approaches. Front Psychiatry. 2022;13:867484.
67. Wu C, Ren C, Teng Z, et al. Cerebral glucose metabolism in bipolar disorder: a voxel-based meta-analysis of positron emission tomography studies. Brain Behav. 2021;11(5):e02117.
68. Ino H, Honda S, Yamada K, et al. Glutamatergic neurometabolite levels in bipolar disorder: a systematic review and meta-analysis of proton magnetic resonance spectroscopy studies. Biol Psychiatry Cogn Neurosci Neuroimaging. 2023;8(2):140-150.
69. Ouyang X, Wang Z, Luo M, et al. Ketamine ameliorates depressive-like behaviors in mice through increasing glucose uptake regulated by the ERK/GLUT3 signaling pathway. Sci Rep. 2021;11(1):18181.
70. Chen MH, Li CT, Lin WC, et al. Persistent antidepressant effect of low-dose ketamine and activation in the supplementary motor area and anterior cingulate cortex in treatment-resistant depression: a randomized control study. J Affect Disord. 2018;225:709-714.
71. Nugent AC, Diazgranados N, Carlson PJ, et al. Neural correlates of rapid antidepressant response to ketamine in bipolar disorder. Bipolar Disord. 2014;16(2):119-128.
72. Williams NR, Heifets BD, Blasey C, et al. Attenuation of antidepressant effects of ketamine by opioid receptor antagonism. Am J Psychiatry. 2018;175(12):1205-1215.
73. Zheng W, Zhou YL, Liu WJ, et al. A preliminary study of adjunctive ketamine for treatment-resistant bipolar depression. J Affect Disord. 2020;275:38-43.
74. Apfel CC, Zhang K, George E, et al. Transdermal scopolamine for the prevention of postoperative nausea and vomiting: a systematic review and meta-analysis. Clin Ther. 2010;32(12):1987-2002.
75. Ellis JS, Zarate CAJ, Luckenbaugh DA, Furey ML. Antidepressant treatment history as a predictor of response to scopolamine: clinical implications. J Affect Disord. 2014;162:39-42.
76. Davis KL, Berger PA, Hollister LE, Defraites E. Physostigmine in mania. Arch Gen Psychiatry. 1978;35(1):119-122.
77. Cannon DM, Carson RE, Nugent AC, et al. Reduced muscarinic type 2 receptor binding in subjects with bipolar disorder. Arch Gen Psychiatry. 2006;63(7):741-747. doi:10.1001/archpsyc.63.7.741
78. Takeuchi I, Suzuki T, Kishi T, et al. Effect of scopolamine butylbromide on clozapine-induced hypersalivation in schizophrenic Patients: a case series. Clin Psychopharmacol Neurosci. 2015;13(1):109-112.
79. Scopolamine in bipolar depression. https://classic.clinicaltrials.gov/ct2/show/NCT04211961. Accessed March 18, 2024.
80. Miravalles C, Kane R, McMahon E, et al. Efficacy and safety of scopolamine compared to placebo in individuals with bipolar disorder who are experiencing a depressive episode (SCOPE-BD): study protocol for a randomised double-blind placebo-controlled trial. Trials. 2022;23(1):339.
81. Smith CM, Santalucia M, Bunn H, Muzyk A. Sublingual dexmedetomidine for the treatment of agitation in patients with schizophrenia and bipolar disorder. Clin Psychopharmacol Neurosci. 2023;21(2):215-221.
82. Preskorn SH, Zeller S, Citrome L, et al. Effect of sublingual dexmedetomidine vs placebo on acute agitation associated with bipolar disorder: a randomized clinical trial. JAMA. 2022;327(8):727-736.
83. Citrome L, Risinger R, Rajachandran L, Robison H. Sublingual dexmedetomidine for agitation associated with schizophrenia or bipolar disorder: a post hoc analysis of number needed to treat, number needed to harm, and likelihood to be helped or harmed. Adv Ther. 2022;39(10):4821-4835.
84. Zareifopoulos N, Panayiotakopoulos G. Treatment options for acute agitation in psychiatric patients: theoretical and empirical evidence. Cureus. 2019;11(11):e6152.
85. Igalmi (dexmedetomidine). Prescribing information. www.fda.gov/medwatch. Accessed February 20, 2024.
86. Kane JM, Harary E, Eshet R, et al. Efficacy and safety of TV-46000, a long-acting, subcutaneous, injectable formulation of risperidone, for schizophrenia: a randomised clinical trial in the USA and Bulgaria. Lancet Psychiatry. 2023;10(12):934-943.
87. Markowicz M, Kubisiak M, Asendrych-Wicik K, et al. Long-acting injectable antipsychotics—a review on formulation and in vitro dissolution. Pharmaceutics. 2023;16:28.
88. Nanaki S, Barmpalexis P, Papakonstantinou Z, et al. Preparation of new risperidone depot microspheres based on novel biocompatible poly(alkylene adipate) polyesters as long-acting injectable formulations. J Pharm Sci. 2018;107(11):2891-2901.
89. Rykindo (risperidone). Prescribing information. Accessed February 14, 2024. www.fda.gov/medwatch. Accessed February 20, 2024.
90. Yunusa I, El Helou ML. The use of risperidone in behavioral and psychological symptoms of dementia: a review of pharmacology, clinical Evidence, regulatory approvals, and off-label use. Front Pharmacol. 2020;11:596.
91. Chiang KJ, Tsai JC, Liu D, et al. Efficacy of cognitive-behavioral therapy in patients with bipolar disorder: a meta-analysis of randomized controlled trials. PLoS One. 2017;12(5):e0176849.
92. Lam DH, Watkins ER, Hayward P, et al. A randomized controlled study of cognitive therapy for relapse prevention for bipolar affective disorder: outcome of the first year. Arch Gen Psychiatry. 2003;60(2):145-152.
93. Frank E. Treating Bipolar Disorder: A Clinician’s Guide to Interpersonal and Social Rhythm Therapy (Series Guide to Individualized Evidence-Based Treatment). Persons JB, ed. 2007.
94. Frank E, Kupfer DJ, Thase ME, et al. Two-year outcomes for interpersonal and social rhythm therapy in individuals with bipolar I disorder. Arch Gen Psychiatry. 2005;62(9):996-1004.
95. Zengin G, Topak OZ, Atesci O, Culha Atesci F. The efficacy and safety of transcranial magnetic stimulation in treatment-resistant bipolar depression. Psychiatr Danub. 2022;34(2):236-244.
96. Brunelin J, Jalenques I, Trojak B, et al. The efficacy and safety of low frequency repetitive transcranial magnetic stimulation for treatment-resistant depression: the results from a large multicenter French RCT. Brain Stimul. 2014;7(6):855-863.
97. Kaiser J, Nay K, Horne CR, et al. CaMKK2 as an emerging treatment target for bipolar disorder. Mol Psychiatry. 2023;(August):1-12.
98. Scott JW, Park E, Rodriguiz RM, et al. Autophosphorylation of CaMKK2 generates autonomous activity that is disrupted by a T85S mutation linked to anxiety and bipolar disorder. Sci Rep. 2015;5:14436.
99. Akarsu S, Bolu A, Aydemir E, et al. The relationship between the number of manic episodes and oxidative stress indicators in bipolar disorder. Psychiatry Investig. 2018;15(5):514-519.
100. Huang W, Liu Y, Luz A, et al. Calcium/calmodulin dependent protein kinase kinase 2 regulates the expansion of tumor-induced myeloid-derived suppressor cells. Front Immunol. 2021;12:754083.
101. Kuloglu M, Ustundag B, Atmaca M, et al. Lipid peroxidation and antioxidant enzyme levels in patients with schizophrenia and bipolar disorder. Cell Biochem Funct. 2002;20(2):171-175.
102. Cudney LE, Sassi RB, Behr GA, et al. Alterations in circadian rhythms are associated with increased lipid peroxidation in females with bipolar disorder. Int J Neuropsychopharmacol. 2014;17(5):715-722.
103. Ying L, Li N, He Z, et al. Fibroblast growth factor 21 ameliorates diabetes-induced endothelial dysfunction in mouse aorta via activation of the CaMKK2/AMPKα signaling pathway. Cell Death Dis. 2019;10(9):665.
104. Versace A, Andreazza AC, Young LT, et al. Elevated serum measures of lipid peroxidation and abnormal prefrontal white matter in euthymic bipolar adults: toward peripheral biomarkers of bipolar disorder. Mol Psychiatry. 2014;19(2):200-208.
105. Cuadrado A, Manda G, Hassan A, et al. Transcription factor Nrf2 as a therapeutic target for chronic diseases: A systems medicine approach. Pharmacol Rev. 2018;70(2):348-383.
106. Wang S, Yi X, Wu Z, et al. CAMKK2 defines ferroptosis sensitivity of melanoma cells by regulating AMPK‒NRF2 pathway. J Invest Dermatol. 2022;142(1):189-200.e8.
107. Ketalar (ketamine hydrochlroide). Prescribing information. Accessed February 22, 2024. www.fda.gov/medwatch.
108. Transderm Scop (scopolamine transdermal system). Prescribing Information. www.fda.gov/Safety/MedWatch. Accessed February 22, 2024.
109. Igalmi (dexmedetomidine). Prescribing information. www.fda.gov/medwatch. Accessed February 22, 2024.
110. Risperidal (risperidone). Prescribing information. www.fda.gov/medwatch. Accessed February 22, 2024.
111. Cognitive behavioral therapy–Mayo Clinic. www.mayoclinic.org/tests-procedures/cognitive-behavioral-therapy/about/pac-20384610. Accessed February 22, 2024.
112. Transcranial magnetic stimulation–Mayo Clinic. www.mayoclinic.org/tests-procedures/transcranial-magnetic-stimulation/about/pac-20384625. Accessed February 22, 2024.
113. CPNP Foundation, Health NA on M. Characterizing the relationship between individuals with mental health conditions and community pharmacists; 2012. www.nami.org. Accessed February 21, 2024.
114. Gianfrancesco FD, Sajatovic M, Rajagopalan K, Wang RH. Antipsychotic treatment adherence and associated mental health care use among individuals with bipolar disorder. Clin Ther. 2008;30(7):1358-1374.
115. Swartz HA, Fagiolini A. Cardiovascular disease and bipolar disorder: risk and clinical implications. J Clin Psychiatry. 2012;73(12):1563-1565.
116. Jawad I, Watson S, Haddad PM, et al. Medication nonadherence in bipolar disorder: a narrative review. Ther Adv Psychopharmacol. 2018;8(12):349-363.
117. Kemp DE. Managing the side effects associated with commonly used treatments for bipolar depression. J Affect Disord. 2014;169(Suppl):S34-S44.
118. Lithobid (lithium carbonate). Prescribing information. www.fda.gov/medwatch. Accessed February 14, 2024.
119. Divalproex (sodium valproate). Prescribing information. www.fda.gov/medwatch. Accessed February 13, 2024.
120. Equetro (carbamazepine). Prescribing information. www.fda.gov/medwatch. Accessed February 14, 2024.
121. Symbyax (olanzapine/fluoxetine). Prescribing information. www.fda.gov/medwatch. Accessed February 14, 2024.
122. Latuda (lurasidone). Prescribing information. www.fda.gov/medwatch. Accessed February 13, 2024.
123. Titulaer J, Radhe O, Danielsson K, et al. Lumateperone-mediated effects on prefrontal glutamatergic receptor-mediated neurotransmission: a dopamine D(1) receptor dependent mechanism. Eur Neuropsychopharmacol. 2022;62:22-35.
The content contained in this article is for informational purposes only. The content is not intended to be a substitute for professional advice. Reliance on any information provided in this article is solely at your own risk.
To comment on this article, contact rdavidson@uspharmacist.com.





