US Pharm. 2017;42(6):47-48.
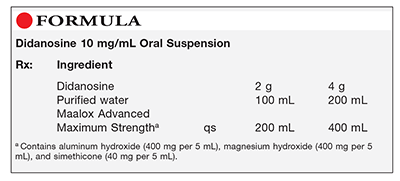
Method of Preparation: Calculate the quantity of each ingredient for the amount to be prepared. Accurately weigh or measure each ingredient. Reconstitute the commercial dry powder to an initial concentration of 20 mg per mL by adding 100 mL or 200 mL of purified water to the 2 g or 4 g of didanosine (Videx) powder, respectively, in the product bottle. Immediately mix one part of the 20 mg per mL initial solution with one part of any commercially available antacid containing aluminum hydroxide (400 mg per 5 mL), magnesium hydroxide (400 mg per 5 mL), and simethicone (40 mg per 5 mL) for a final dispensing concentration of 10 mg didanosine (Videx) per mL. Package and label.
Use: Videx (didanosine, USP; also known as ddI), in combination with other antiretroviral agents, is indicated for the treatment of HIV-1 infection.
Packaging: For home use by patients, the preparation should be dispensed in appropriately sized flint-glass or plastic (high-density polyethylene [HDPE], poly-ethylene terephthalate [PET], or polyethylene terephthalate glycol [PETG]) bottles with child-resistant closures.
Labeling: Keep out of reach of children. Keep refrigerated. Shake well. Discard after ____ [time period].
Stability: The Videx admixture may be stored for up to 30 days in a refrigerator at 36°F to 46°F (2°C to 8°C). Discard any unused portion after 30 days.1,2
Quality Control: Quality-control assessment can include weight/volume, pH, specific gravity, active drug assay, color, rheologic properties/pourability, physical observation, and physical stability (discoloration, foreign materials, gas formation, mold growth).3
Discussion: This preparation is a good example of how pharmacists can be involved in managing patients’ therapy. The package insert states that the patient should read the Videx Medication Guide before starting to take Videx, and again each time the prescription is refilled, to check for new information. This should take place with the pharmacist at the time of dispensing (original and refills). Printed information does not take the place of talking with one’s healthcare provider about the medical condition or treatment. The patient should stay under a healthcare provider’s care when taking Videx.
Didanosine is a nucleoside reverse transcriptase inhibitor for use in combination with other antiretroviral agents for the treatment of HIV-1 infection. Videx is a brand name for didanosine, USP, a synthetic purine nucleoside analogue active against HIV-1. Didanosine is available as Videx, a pediatric powder for oral solution.
Didanosine occurs as a white, crystalline powder with the molecular formula C10H12N4O3 and a molecular weight of 236.2. The aqueous solubility of didanosine at 25°C and a pH of approximately 6 is 27.3 mg per mL. Didanosine is unstable in acidic solutions. For example, at a pH of <3 and a temperature of 37°C, 10% of didanosine decomposes to hypoxanthine in less than 2 minutes. This is the reason didanosine is to be administered on an empty stomach and is combined with an antacid mixture.
Videx (didanosine, USP) Pediatric Powder for Oral Solution is supplied in 4-oz. and 8-oz. glass bottles containing 2 g or 4 g of Videx, respectively. Regarding pediatric dosing, for patients aged 2 weeks to 18 years, this product is administered on an empty stomach at least 30 minutes before or 2 hours after eating. Specific pediatric dosages are as follows:
• Between age 2 weeks and 8 months, the dosing is 100 mg per m2 twice daily.
• For patients older than 8 months, the dosing is 120 mg per m2 twice daily, but not to exceed the adult dosing recommendation.
Maalox Advanced Maximum Strength contains aluminum hydroxide (400 mg per 5 mL), magnesium hydroxide (400 mg per 5 mL), and simethicone (40 mg per 5 mL) as active ingredients, as well as the following inactive ingredients: butylparaben, carboxymethyl cellulose sodium, flavor, glycerin, hypromellose, microcrystalline cellulose, potassium citrate, propylene glycol, propylparaben, purified water, saccharin sodium, and sorbitol.4
REFERENCES
1. U.S. Pharmacopeia/National Formulary [current revision]. Rockville, MD: U.S. Pharmacopeial Convention, Inc; May 2017.
2. Videx (didanosine powder for solution) package insert. Princeton, NJ: Bristol-Myers Squibb Co; August 2015.
3. Allen LV Jr. Standard operating procedure for performing physical quality assessment of oral and topical liquids. IJPC. 1999;3:146-147.
4. Maalox. www.maaloxus.com/?maxLiquid. Accessed May 6, 2017.
To comment on this article, contact rdavidson@uspharmacist.com.





