US Pharm. 2018;43(8)(Specialty&Oncology suppl):2-11.
ABSTRACT: Because cancer patients are at high risk for various thrombotic complications, treatment or prophylaxis with an appropriate anticoagulant is often necessary. The direct oral anticoagulants (DOACs) rivaroxaban, apixaban, edoxaban, betrixaban, and dabigatran offer a unique alternative to warfarin, unfractionated heparin, and low-molecular-weight heparin for the treatment of a variety of disease states. The use of DOACs in cancer patients is an area of interest because of the drugs’ convenience and effectiveness in the general population. New evidence for DOAC use in cancer patients is emerging, suggesting that these medications may be used safely and effectively in this population.
Twenty percent of new cases of venous thromboembolism (VTE) are associated with an underlying cancer.1 Likewise, the risk of atrial fibrillation (AF), and consequently stroke, is increased in patients with malignancy.2 The prevention and treatment of VTE and stroke associated with AF in cancer patients are, therefore, important considerations. Apixaban, betrixaban, dabigatran, edoxaban, and rivaroxaban constitute the direct oral anticoagulants (DOACs). These drugs, which were FDA-approved within the last decade, offer an alternative means of anticoagulation to vitamin K antagonists (VKAs), unfractionated heparin, and low-molecular-weight heparin (LMWH). Indications for DOAC use include stroke prophylaxis in nonvalvular AF and treatment and prophylaxis in deep venous thrombosis and pulmonary embolism.3 Beyond the use of DOACs for these diseases in the setting of malignancy, the prospect of additional therapeutic options for anticoagulation in cancer patients is intriguing. Cancer patients pose unique challenges because their weight-based and renally based dosing needs vary, they are hypercoagulable at baseline, and they have been underrepresented in most DOAC trials.4
Technical Considerations
One of the primary challenges in patients requiring long-term anticoagulation is the transition from inpatient to outpatient management. This is because of traditional therapies’ limitations for both patients and clinicians. LMWH is dosed based on body weight and requires subcutaneous—and sometimes twice daily—administration, which cancer patients may struggle with because of frequency, fear of injections, and side effects at the injection site, such as bleeding or bruising. VKAs are available orally, but they require a stringent monitoring regimen. Many factors can lead to variations in international normalized ratio with the use of VKAs, including concurrent medications, diet, and alcohol consumption. Most of these factors fluctuate in the oncology population, thereby decreasing predictability of therapy.5
In the CLOT trial, cancer patients at various stages of progression and with clinical VTE received either LMWH or a VKA for 6 months. No difference in mortality was shown initially, but a post hoc analysis was performed to determine whether there was a mortality difference among patients without metastatic disease. In that group, it was found that death occurred 50% less in patients receiving LMWH than those receiving VKA therapy.6 Because DOACs are more closely related mechanistically to LMWH than to VKAs, they could potentially offer a useful oral alternative for cancer patients while retaining efficacy. This outcome was corroborated by a large meta-analysis of patients on anticoagulation for both VTE and AF in which DOACs were associated with half as many fatal bleeds as warfarin.7 It must be noted, however, that specific reversal agents are commercially available only for dabigatran, rivaroxaban, and apixaban at this time.8,9
Review of Available Evidence
Cancer constitutes a unique coagulopathy whose pathogenesis is multifactorial and includes the expression of adhesion molecules and hemostatic proteins and the production of inflammatory cytokines, microparticles, and proangiogenic factors.10 Recommendations for anticoagulation in cancer patients can differ from those for the general population. TABLE 1 summarizes the most recent guideline recommendations for common indications for anticoagulation, both in the general population and in oncology patients.
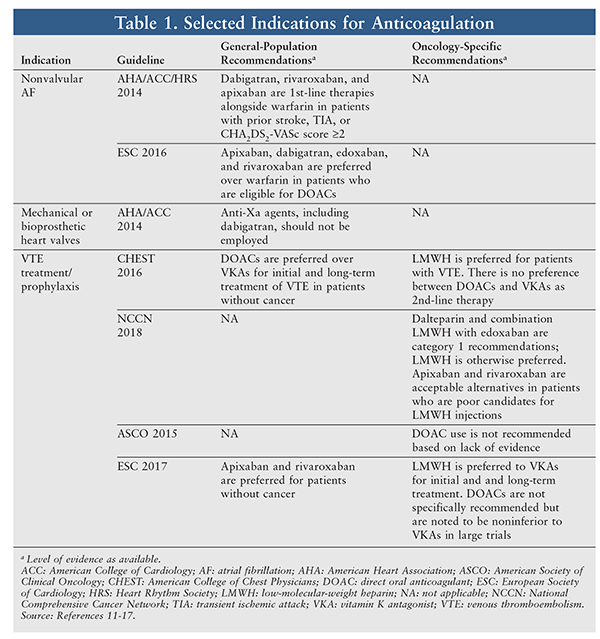
For stroke prevention in nonvalvular AF, DOACs are first-line therapy in both the American Heart Association and European Society of Cardiology (ESC) guidelines.11,12 Neither guideline has specific recommendations for cancer patients. Patients with bioprosthetic or mechanical heart valves who require anticoagulation should not receive a DOAC because of the potential for increased risk of thromboembolism and bleeding.13 For the treatment of VTE, DOACs are preferred in noncancer patients, whereas LMWH is preferred in cancer patients by both the American College of Chest Physicians and the ESC.14,15
The role ascribed to DOACs by oncology-specific societies varies. The 2015 update to the American Society of Clinical Oncology guideline recommends against DOAC use based on a lack of evidence.16 However, the 2018 update to the National Comprehensive Cancer Network (NCCN) guideline for VTE management includes increased incorporation of DOACs as therapeutic options.17 The combination of parenteral anticoagulation for 5 to 10 days with maintenance edoxaban is newly listed by the NCCN as a category 1 recommendation (dalteparin alone is the only other category 1 pharmacotherapy). Among remaining therapies, LMWH is preferred, but apixaban monotherapy, rivaroxaban monotherapy, and the combination of at least 5 days of parenteral anticoagulation with maintenance dabigatran have been added as alternatives for patients with compelling reasons to avoid LMWH (e.g., injection-related pain, affordability, or inconvenience).17
Overall, there is a paucity of data surrounding the use of DOACs in cancer patients. Prior to 2018, no published prospective studies primarily addressed DOAC use in cancer patients. Historically, most data originated from subgroup analyses of a number of larger studies involving both noncancer patients and cancer patients. In all reported trials, safety and efficacy did not differ greatly between DOAC and non-DOAC treatment groups (TABLE 2). In some studies, such as AMPLIFY and that performed by Levine and colleagues, the use of DOACs constituted a significant improvement over comparator therapies.18,19 Major limitations of the described literature are that most subgroups of cancer patients were small and results in cancer patients had limited applicability to general practice.
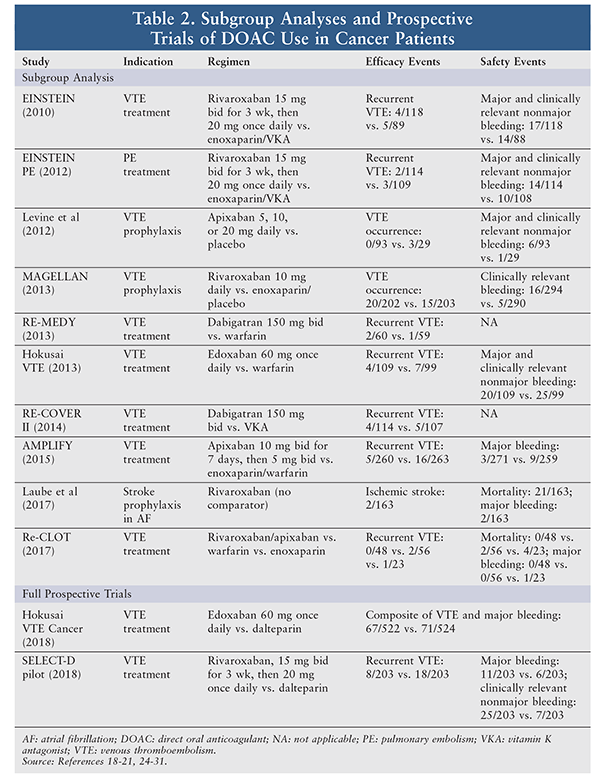
The Hokusai VTE Cancer Investigators conducted the first prospective trial of DOAC use in cancer patients.20 This randomized, open-label trial compared the efficacy and safety of edoxaban versus dalteparin in the treatment of 1,046 cancer patients with VTE. In the trial, edoxaban was found to be noninferior for the composite endpoint of recurrent VTE or major bleeding (hazard ratio [HR] 0.97; 95% CI 0.70-1.36, P = .006). Major bleeding was higher in the edoxaban group (HR 1.77; 95% CI 1.03-3.04, P = .04). Notably, there was an increased rate of major bleeds in edoxaban patients with gastrointestinal (GI) cancers compared with the dalteparin group (13.2% vs. 2.4%, P = .0169). Additionally, at randomization, more patients in the edoxaban group than in the dalteparin group had GI cancer (26.1% vs. 23.9%). The higher rate of bleeding found in patients with GI cancer in the Hokusai VTE Cancer trial suggests that cancer patients are not a homogenous cohort in terms of bleeding risk with DOACs. Therefore, further research into specific subpopulations may be warranted to explore DOAC use in specific cancers.
SELECT-D was a prospective, randomized, open-label pilot study with goals similar to those of the Hokusai VTE Cancer trial.21 In SELECT-D, the efficacy and safety of rivaroxaban versus dalteparin were compared for VTE treatment in 406 patients with cancer. It was found that the cumulative incidence of VTE recurrence at 6 months was lower with rivaroxaban (4% vs. 11%; HR 0.43; 95% CI 0.19-0.99). There was no clinically significant difference in rates of major bleeding, but a higher rate of clinically relevant nonmajor bleeding was observed in patients taking rivaroxaban (13% vs. 4%; HR 3.76; 95% CI 1.63-8.69). A similar increase in DOAC-related bleeding for esophageal and gastroesophageal cancer patients was again noted, with 11 rivaroxaban patients and only four dalteparin patients experiencing a major bleed. This lends further credibility to the hypothesis that cancer patients are not a homogenous cohort with respect to bleeding risk.
Future Research
A number of trials are investigating the use of DOACs for both prevention and treatment of VTE in cancer patients. ADAM-VTE is a phase IV randomized, open-label trial that is assessing rates of major bleeding for apixaban compared with dalteparin when used for VTE treatment.22 CASSINI is a phase IIIb randomized, controlled trial that is investigating first occurrence of VTE in high-risk ambulatory cancer patients receiving rivaroxaban versus placebo.23 Both of these trials offer the prospect of higher-quality evidence that has the potential to guide future therapy.
The Pharmacist’s Role
In the setting of emerging evidence, pharmacists can play a crucial role in the management of anticoagulation in cancer patients. Prescribers may not be aware of the differences between individual DOAC agents in terms of dosing, cost, and administration requirements. When anticoagulation is necessary in a cancer patient, the pharmacist can help reconcile such factors with the quality of data available for individual agents and can recommend the safest, most effective therapy. Likewise, the pharmacist can help determine whether DOAC therapy is appropriate based on the patient’s risk factors (e.g., presence of GI cancer, obese habitus, severe renal dysfunction).
Conclusion
DOACs are a convenient method of therapy for many patients, but appropriate use in cancer patients has been poorly defined until recently. Many high-profile consensus statements offer limited guidance on the use of DOACs in oncology patients; however, a growing body of literature suggests that DOACs may be used safely and effectively in this population. Emerging evidence suggests that DOACs may be considered in cancer patients with limitations in using LMWH or VKAs, and overall DOAC use in these patients will be reviewed as upcoming trial data become available.
REFERENCES
1. Wun T, White RH. Epidemiology of cancer-related venous thromboembolism. Best Pract Clin Haematol. 2009;22:9-23.
2. Fitzpatrick T, Carrier M, Le Gal G. Cancer, atrial fibrillation, and stroke. Thromb Res. 2017;155:101-105.3. Saljoughian M. Assessing novel oral anticoagulants. US Pharm. 2018;43(2):13-14.4. Bauersachs RM. LMWH in cancer patients with renal impairment—better than warfarin? Thromb Res. 2016;140(suppl 1):S160-S164.5. Falanga A, Zacharski L. Deep vein thrombosis in cancer: the scale of the problem and approaches to management. Ann Oncol. 2005;16:696-701.6. Lee AY, Levine MN, Baker RI, et al. Low-molecular-weight heparin versus a coumarin for the prevention of recurrent venous thromboembolism in patients with cancer. N Engl J Med. 2003;349:146-153.7. Chai-Adisaksopha C, Hillis C, Isayama T, et al. Mortality outcomes in patients receiving direct oral anticoagulants: a systematic review and meta-analysis of randomized controlled trials. J Thromb Haemost. 2015;13:2012-2020.8. Praxbind (idarucizumab) package insert. Ridgefield, CT: Boehringer Ingelheim Pharmaceuticals, Inc; October 2015.
9. Andexxa (coagulation factor Xa [recombinant], inactivated-zhzo) package insert. South San Francisco, CA: Portola Pharmaceuticals, Inc; 2018.
10. Falanga A, Russo L, Milesi V. The coagulopathy of cancer. Curr Opin Hematol. 2014;21:423-429.11. January CT, Wann LS, Alpert JS, et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines and the Heart Rhythm Society. Circulation. 2014;130:e199-e267.12. Kirchhof P, Benussi S, Kotecha D, et al. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur J Cardiothorac Surg. 2016;50:e1-e88.13. Nishimura RA, Otto CM, Bonow RO, et al. 2014 AHA/ACC guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63:e57-e185.14. Kearon C, Akl EA, Ornelas J, et al. Antithrombotic therapy for VTE disease: CHEST guideline and expert panel report. Chest. 2016;149:315-352.15. Mazzolai L, Aboyans V, Ageno W, et al. Diagnosis and management of acute deep vein thrombosis: a joint consensus document from the European Society of Cardiology working groups of aorta and peripheral circulation and pulmonary circulation and right ventricular function. Eur Heart J. 2017 Feb 17 [Epub ahead of print].16. Lyman GH, Bohlke K, Khorana AA, et al. Venous thromboembolism prophylaxis and treatment in patients with cancer: American Society of Clinical Oncology clinical practice guideline update 2014. J Clin Oncol. 2015;33:654-656.17. National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines). Cancer-associated venous thromboembolic disease. Version 1.2018. www.nccn.org/professionals/physician_gls/pdf/vte.pdf. Accessed June 25, 2018.
18. Agnelli G, Buller HR, Cohen A, et al. Oral apixaban for the treatment of venous thromboembolism in cancer patients: results from the AMPLIFY trial. J Thromb Haemost. 2015;13:2187-2191.19. Levine MN, Gu C, Liebman HA, et al. A randomized phase II trial of apixaban for the prevention of thromboembolism in patients with metastatic cancer. J Thromb Haemost. 2012;10:807-814.20. Raskob GE, van Es N, Verhamme P, et al. Edoxaban for the treatment of cancer-associated venous thromboembolism. N Engl J Med. 2018;378:615-624.21. Young AM, Marshall A, Thirlwall J, et al. Comparison of an oral factor xa inhibitor with low molecular weight heparin in patients with cancer with venous thromboembolism: results of a randomized trial (SELECT-D). J Clin Oncol. 2018 May 10: JCO2018788034 [Epub ahead of print].22. McBane Ii R, Loprinzi CL, Ashrani A, et al. Apixaban and dalteparin in active malignancy associated venous thromboembolism. The ADAM VTE Trial. Thromb Haemost. 2017;117:1952-1961.23. Khorana AA, Vadhan-Raj S, Kuderer NM, et al. Rivaroxaban for preventing venous thromboembolism in high-risk ambulatory patients with cancer: rationale and design of the CASSINI trial. Thromb Haemost. 2017;117:2135-2145.24. Bauersachs R, Berkowitz SD, Brenner B, et al. Oral rivaroxaban for symptomatic venous thromboembolism. N Engl J Med. 2010;363:2499-2510.25. Büller HR, Prins MH, Lensin AW, et al. Oral rivaroxaban for the treatment of symptomatic pulmonary embolism. N Engl J Med. 2012;366:1287-1297.26. Cohen AT, Spiro TE, Büller HR, et al. Rivaroxaban for thromboprophylaxis in acutely ill medical patients. N Engl J Med. 2013;368:513-523.27. Schulman S, Kearon C, Kakkar AK, et al. Extended use of dabigatran, warfarin, or placebo in venous thromboembolism. N Engl J Med. 2013;368:709-718.28. Büller HR, Décousus H, Grosso MA, et al. Edoxaban versus warfarin for the treatment of symptomatic venous thromboembolism. N Engl J Med. 2013;369:1406-1415.29. Schulman S, Kakkar AK, Goldhaber SZ, et al. Treatment of acute venous thromboembolism with dabigatran or warfarin and pooled analysis. Circulation. 2014;129:764-772.30. Laube ES, Yu A, Gupta D, et al. Rivaroxaban for stroke prevention in patients with nonvalvular atrial fibrillation and active cancer. Am J Cardiol. 2017;120:213-217.31. Alzghari SK, Seago SE, Garza JE, et al. Retrospective comparison of low molecular weight heparin vs. warfarin vs. oral Xa inhibitors for the prevention of recurrent venous thromboembolism in oncology patients: the Re-CLOT study. J Oncol Pharm Pract. 2017 Jan 1: 1078155217718382 [Epub ahead of print].
To comment on this article, contact rdavidson@uspharmacist.com.





