US Pharm. 2020;45(6):30-32.
ABSTRACT: The FDA defines a generic drug as a “medication created to be the same as an already marketed brand-name drug in dosage form, safety, strength, route of administration, quality, performance characteristics, and intended use.” The U.S. healthcare system saved almost $2 trillion from 2009 to 2019 in healthcare costs due to generic use, with increased savings every year. Despite this variation in cost, barriers such as lack of communication and education, previous side effects, or preference may play a role in continued use of brand-name medications. Having an increased generic product market will create competition and, ultimately, lead to lower drug prices and access to affordable healthcare for more patients. It is important that pharmacists educate patients and providers on the appropriate use of generic versus brand medications.
The FDA defines a generic drug as a “medication created to be the same as an already marketed brand-name drug in dosage form, safety, strength, route of administration, quality, performance characteristics, and intended use.”1,2 Generic medications, therefore, are equivalent to brand-name products in terms of efficacy, quality, and safety. Abbreviated New Drug Applications (ANDAs) for generic approvals must show bioequivalence and that the active ingredient is the same as the brand name; they may differ based on the inactive ingredients, but these differences must be proven to have no effect on how the medication works.1-3
The 1984 Drug Price Competition and Patent Term Restoration Act, or Hatch-Waxman Act, allowed for generics to be approved for market use without preclinical and clinical testing to ensure a generic would be lower in cost compared with its equivalent branded product. Generics range from 80% to 85% lower in cost when compared with their brand product.1 According to the IQVIA 2019 data, the U.S. healthcare system saved almost $2 trillion from 2009 to 2019 due to generic usage, with increased savings every year.4,5 According to a report by the Association of Accessible Medicines (AAM), 4 billion generic prescriptions were filled in 2018 in the U.S., which represented 90% of prescriptions filled in the U.S. However, this only accounted for 22% of all drug spending.5
Due to perceptions or past experiences with generic medications, some consumers continue to use brand-name medications despite this difference in cost, which may lead to an increase in consumer spending, patient dissatisfaction, and ultimately, a decrease in adherence. Answering questions or discussing the use of generic versus brand medications or inactive versus active ingredients with patients is an important role for pharmacists to help improve patient satisfaction, adherence, and quality of life. Having an increased market of generic products will create competition and, ultimately, lead to lower drug prices and access to affordable healthcare for more patients.1
Discussion Points
Due to misconceptions and lack of understanding of the difference between brand and generic products, educating patients about the facts is vital. Outlined in the next few paragraphs are concepts of discussion that may be important to our patients.
What Is the Difference Between Active and Inactive Ingredients?
An active ingredient is the component of the drug that produces the pharmacologic effect on the body. Active ingredients may undergo chemical changes as the product was manufactured and be present in a modified form for it to provide its effect.6 An example of this would be an extended-release formulation of a medication. An inactive ingredient is a component of the drug that does not affect the therapeutic action of the medication on the body. Inactive ingredients are added to a drug product to help with binding, flavoring, coloring, preserving, or drug transport. Select examples of inactive ingredients are alcohol, gelatin, galactose, lactose, and saccharin.
To gain FDA approval, a generic medication must contain the same active ingredient as the originator medication (i.e., brand-name product). Although the inactive ingredients may be different, the FDA regularly conducts extensive reviews of the generic products to ensure they meet standards. The FDA understands that there may be variability in each batch made, but the amount of variability is limited. This variability can occur between branded medications and generics, but it also can take place between brands as well, due to slight differences in batch manufacturing.1 The FDA also investigates reported side effects with a brand or generic product to determine if manufacturing changes are needed.
What Is the Difference Between a Generic Product and an Authorized Generic?
A generic product is the equivalent of a branded product made by a company separate from the company that makes the brand product. An authorized generic, however, may be marketed by the brand-name drug company or company with the brand’s permission but as a generic at a lower cost. Authorized generics are the same as the brand-name drug in that they have the same active and inactive ingredients, but their appearance may differ.7 An authorized generic may not be listed in the FDA’s listing of generic medications, known as the Orange Book, since it is marketed under the brand’s New Drug Application versus generic products, which are approved after submission of an ANDA.7,8
Both generics and authorized generics may be lower in cost when compared with branded medications since generic manufacturers do not have to complete the clinical trials that were required for branded products.
Is There Anything to Look Out for When Switching From a Brand to a Generic Product?
Brand and generic medications are not allowed to look the same, due to trademark laws in the U.S. Differences in color, shape, size, and flavor, however, do not play a role in the way the medications work. As previously stated, variability does occur in batches but at very small, nonsignificant levels. For example, a large study comparing generics with brand-name medicines found small differences of only 3.5% in absorption into the body between generic and brand medicines.9
Possible Barriers to Generic Prescribing and Ways to Overcome
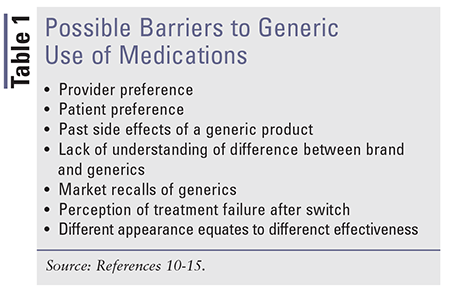
Surveys and studies have been conducted throughout the years to analyze the barriers in generic usage or increase in switchbacks from generic to brand. TABLE 1 provides a list of possible barriers to generic prescribing and dispensing.10-15
A survey conducted in epileptic patients and their concerns of generic substitutions concluded that patients believed that changes should only be determined by the provider or patient. These concerns stemmed from providers’ concerns of breakthrough seizure when switching or increased risk of side effects and from patients’ concerns that generics may not provide equivalent efficacy or tolerability.16 However, it is also been shown that having a relationship with one’s pharmacist and making provider and patients aware of manufacturer changes may help improve generic usage in epileptic patients.17
In addition, some clinicians and scientists have expressed concerns about the FDA’s bioequivalence approval. However, the Therapeutic Inequivalence Action Coordinating Committee, a postmarketing review group of the FDA, identifies and evaluates reports of therapeutic failures to determine if removal of inequivalent products is needed.9 In addition, studies have concluded that the bioequivalence testing required by the FDA is robust and helps support the approval that brand and generics are therapeutically equivalent.9
Studies have reviewed switchback rates or the amount of times that patients switch back to branded drugs from generics despite a cost differential. Studies reviewing authorized generics and generics showed a lower rate of brand switchbacks due to interventions to improve communication and education on the equivalency of generics and brand-name products.11,12 In addition, similarities in appearance also prevented brand switchbacks.11
Due to recent recalls on generic products, patients may also be apprehensive to switch to generics as they may not be produced in the U.S., believing that medications manufactured outside of the U.S. are not safe, despite FDA approval. Proper education on the details of recalls are effective to encourage adherence to medications.14
Despite the availability of a generic drug, the use of a brand-name product may be clinically appropriate in select situations. For example, previous patients taking epileptic medications who experienced an increase in seizure frequency with a generic switch may be appropriate for a change to a brand-name medication for seizure control.15
With the various barriers discussed above, multiple studies have shown that promoting educational interventions for patients and providers may help increase confidence in generic use.13 In addition, educating on generic use may help improve quality of care and adherence to medications. The 2019 AAM report analyzed the abandonment rates for medications, examining the rate at which patients may not take or pick up their medications despite requesting the prescription. The report concludes that new patient abandonment rates are 266% higher for brand-name drugs when compared with generic drugs.5 Copayments played a significant role in this abandonment rate; 90% of generic copays were under $20 compared with the 39% of brand medications.5
AVAILABLE RESOURCES TO ASSIST WITH EDUCATION
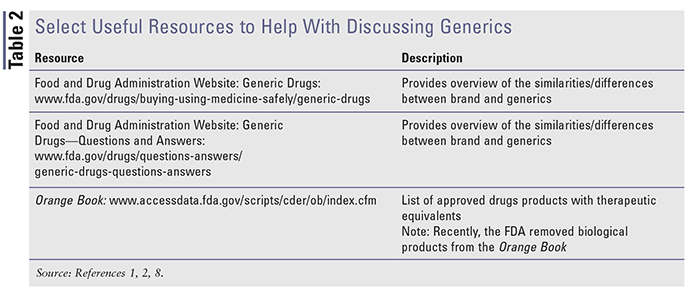
Multiple resources are available to help understand and discuss brand and generics. Please see TABLE 2 for an overview of select useful available resources.
CONCLUSION
As pharmacists, we set forth an oath to apply our knowledge to assure optimal outcomes for our patients and advocate changes that improve patient care. Improving adherence, educating our patients on facts, and having open communication with providers on information is vital for us to fulfill our oath as a pharmacist. The FDA completes a rigorous process to approve generic medications into the U.S. market; however, due to perceptions or amount of viable information, patients may be apprehensive to take generic medications. Studies have proven that overcoming barriers and educating patients and providers on facts of generic versus brand may help improve generic prescribing and ultimately help produce affordable healthcare.
REFERENCES
1. FDA. Generic drugs. www.fda.gov/drugs/buying-using-medicine-safely/generic-drugs. Accessed March 19, 2020.
2. FDA. Generic drugs: questions and answers. www.fda.gov/drugs/questions-answers/generic-drugs-questions-answers. Accessed March 19, 2020.
3. Dunne S, Shannon B, Dunne C, Cullen W. A review of the differences and similarities between generic drugs and their originator counterparts, including economic benefits associated with usage of generic medicines, using Ireland as a case study. BMC Pharmacol Toxicol. 2013;14:1.
4. IQVIA Institute for Human Data Science. Global medicine spending and usage trends: outlook 2024. www.iqvia.com/-/media/iqvia/pdfs/institute-reports/global-medicine-spending-and-usage-trends.pdf?_=1586623006517. Accessed March 20, 2020.
5. Association for Accessible Medicines. The case for competition: 2019 generic drug and biosimilars access and savings in the U.S. report. https://accessiblemeds.org/sites/default/files/2019-09/AAM-2019-Generic-Biosimilars-Access-and-Savings-US-Report-WEB.pdf. Accessed March 20, 2020.
6. FDA. Drug approvals and databases: inactive ingredient. www.fda.gov/drugs/drug-approvals-and-databases/inactive-ingredient-search-approved-drug-products-frequently-asked-questions. Accessed March 20, 2020.
7. FDA. Abbreviated New Drug Application (ANDA): FDA list of authorized generic drugs. www.fda.gov/drugs/abbreviated-new-drug-application-anda/fda-list-authorized-generic-drugs. Accessed March 21, 2020.
8. FDA: Orange Book: approved drug products with therapeutic equivalence evaluations. www.accessdata.fda.gov/scripts/cder/ob/index.cfm. Accessed March 21, 2020.
9. Davit BM, Nwakama PE, Buehler GJ, et al. Comparing generic and innovator drugs: a review of 12 years of bioequivalence data from the United States Food and Drug Administration. Ann Pharmacother. 2009;43(10):1583-1597.
10. Choudhry NK, Denberg D, Qaseem A. Improving adherence to therapy and clinical outcomes while containing costs: opportunities from the greater use of generic medications: best practice advice from the Clinical Guidelines Committee of the American College of Physicians. Ann Intern Med. 2016;164:41-49.
11. Desai RJ, Sarpatwari A, Dejene S, et al. Diff
12. Hansen RA, Qian J, Berg R, et al. Comparison of generic-to-brand switchback rates between generic and authorized generic drugs. Pharmacotherapy. 2017;37(4):429-437.
13. Desai RJ, Sarpatwari A, Dejene S, et al. Comparative effectiveness of generic and brand-name medication use: a database study of US health insurance claims. PLoS Med. 2019;16(3):e1002763.
14. FDA. What to know and do about possible nitrosamines in your medication. www.fda.gov/consumers/consumer-updates/what-know-and-do-about-possible-nitrosamines-your-medication. Accessed May 13, 2020.
15. Straka RJ, Keohane DJ, Liu LZ. Potential clinical and economic impact of switching branded medications to generics. Am J Ther. 2017;24(3):e278-e289.
16. Ngo SNT, Stupans I, McKinnon RA. Generic substitution in the treatment of epilepsy: patient attitudes and perceptions. Epilepsy Behav. 2013;26:64-66.
17. Greb E. Study reveals concerns about switching from branded to generic antiepileptic drugs. Neurology Rev. 2014;22(3):6.
The content contained in this article is for informational purposes only. The content is not intended to be a substitute for professional advice. Reliance on any information provided in this article is solely at your own risk.
To comment on this article, contact rdavidson@uspharmacist.com.






