US Pharm. 2008;33(9):HS11-HS20.
Pharmacists often receive
inquiries about the effects of medications on blood pressure. For example,
concerns about OTC cough and cold products still arise even though some
ingredients, such as phenylpropanolamine, have been removed from the U.S.
market. While only a few classes of drugs cause clinically significant
increases in arterial pressure, pharmacists should be aware of drugs that may
interfere with effective blood pressure control. A review of drug–drug
interactions with antihypertensive agents is beyond the scope of this article.
However, some of the more common examples of drug-induced hypertension will be
discussed (TABLE 1). Drug-induced blood pressure elevations represent
an important and modifiable cause of secondary hypertension; therefore, it is
imperative that pharmacists recognize this causal relationship.
Sympathomimetic Agents
It is well
established that sympathomimetic amines cause dose-related increases in blood
pressure.1-4 While sympathomimetic-induced hypertension may not be
clinically significant in healthy patients, it can become hazardous in others.1-4
Sympathomimetic amines include amphetamines and similar compounds, such as
pseudoephedrine, phenylpropanolamine, and ephedrine. Historically, these
compounds were contained in some OTC cough and cold preparations. Because
phenylpropanolamine use was correlated with hypertension and stroke, the FDA
banned it from the market in November 2000.3,4
Pseudoephedrine:
Pseudoephedrine is a bronchodilator and nasal vasoconstrictor that is
generally innocuous when used in recommended doses. However, due to its
potential for misuse, many retailers restrict its sale to behind the counter.
Pseudoephedrine is commonly used to treat symptoms of rhinitis and rhinorrhea,
but its effects on blood pressure and heart rate remain uncertain. Because of
its pharmacologic similarity to ephedrine and phenylpropanolamine, use of
pseudoephedrine has likewise been avoided in hypertensive patients.
Salerno et al assessed whether
pseudoephedrine causes clinically meaningful elevations in blood pressure and
heart rate.5 In this meta-analysis, the primary data extracted
included systolic and diastolic blood pressure and heart rate. Twenty-four
clinical trials had extractable vital sign information and included a total of
1,285 patients. This analysis demonstrated that pseudoephedrine causes a small
mean increase in systolic blood pressure (approximately 1 mmHg), with no
significant effect on diastolic blood pressure, and a slight increase in
heart rate (about 3 beats per minute). Immediate-release formulations had a
greater effect than sustained-releaseformulations, which would be expected
based on pharmacokinetics. Among immediate-release formulations, there was
a dose-related increase in all three cardiovascular variables. More
substantial increases in both systolic and diastolic blood pressure were noted
with increasing doses of pseudoephedrine. Women seemed to be slightly less
susceptible to the cardiovascular effects than men. In patients
whose hypertension was stable and controlled, pseudoephedrine therapy
increased systolic blood pressure but had no effect on diastolic pressure.
There was no effect on heart rate in treated hypertensive patients, though
this may have been because many patients were receiving beta-blockers. There
was no documentation of any clinically significant adverse outcomes,
such as hypertensive emergencies, stroke, or arrhythmia. Other investigators
have similarly concluded that when it is used at standard doses,
pseudoephedrine does not have a clinically significant effect on systolic or
diastolic blood pressure in patients with controlled hypertension.6
Pharmacists should counsel
patients that pseudoephedrine may modestly increase blood pressure and heart
rate. These effects are greatest with immediate-release formulations,
higher doses, and short-term medication administration. Patients
with stable, controlled hypertension do not seem to be at higher
risk for blood pressure elevation compared to those without hypertension.
However, one cannot predict how any individual patient will react.
The risk-benefit ratio should be evaluated carefully before using
any sympathomimetic agent in persons with hypertension. Pharmacists shouldinstruct
patients with cardiovascular disease to monitor their blood
pressures carefully after starting pseudoephedrine-containing medications.
Sustained-release products would generally be preferred to avoid increases in
blood pressure. Alternatively, intranasal decongestants such as oxymetazoline
could be used, since they have not been shown to induce hypertension when used
at recommended doses.7
Amphetamine Derivates:
A variety of drugs used for narcolepsy and attention-deficit/hyperactivity
disorder are chemically related to amphetamine. These central nervous system
(CNS) stimulants include dextroamphetamine, methamphetamine, and
methylphenidate. The FDA recently issued a warning for dextroamphetamine,
stating that using CNS-stimulant treatment at usual doses in children and
adolescents with serious heart problems and structural cardiac abnormalities
has been associated with sudden death.8 However, adverse
cardiovascular events induced by stimulants are not limited to children.
Adults with known cardiac disease have also shown increased risk of sudden
death with stimulant use at normal doses. As a general rule,
amphetamine-related compounds (i.e., CNS stimulants) should be avoided in
patients with known serious structural cardiac abnormalities, cardiomyopathy,
serious heart rhythm abnormalities, or other serious cardiac problems that
increase the risk of sudden death. Increases in both heart rate and blood
pressure have been observed in children receiving drugs in this class.9
Thus, this potential cardiovascular risk should be balanced against the
beneficial behavioral effects of these medications.
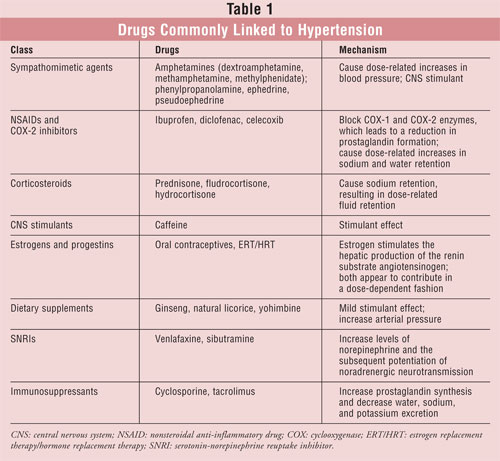
NSAIDs and COX-2 Inhibitors
Nonsteroidal
anti-inflammatory drugs (NSAIDs) have potentially adverse effects on blood
pressure.10,11 NSAIDs block both cyclooxygenase-1 (COX-1) and COX-2
enzymes, which leads to a reduction in prostaglandin formation. These drugs
can have widespread beneficial and harmful effects, depending on the patient
context. Drug-induced hypertension associated with NSAIDs is due to the renal
effects of these drugs. Specifically, NSAIDs cause dose-related increases in
sodium and water retention. This effect is also seen with COX-2 selective
agents, such as celecoxib.11
The COX-1 and COX-2 isoforms
are both expressed within the normal adult kidney, with COX-1 in the
glomerulus and afferent arteriole and COX-2 in the afferent arteriole, the
podocytes, and macula densa.12 The specific location of each of
these isoenzymes in the kidney translates into notably different effects on
renal function. The prostaglandins produced by COX-1 primarily affect renal
homeostasis by promoting vasodilation in the renal vascular bed, reducing
renal vascular resistance, and consequently increasing renal perfusion.
Prostaglandins produced by the COX-2 isoenzyme have diuretic and natriuretic
effects.12,13 In patients who are hemodynamically compromised, the
effects of the two isoenzymes are essential for the maintenance of renal
perfusion because of their vasodilatory effects. Because NSAIDs block the
production of the COX-1 and COX-2 prostaglandins, renal side effects are not
uncommon, occurring in approximately 1% to 5% of NSAID users.13
By inhibiting COX-2's
natriuretic effect, thereby increasing sodium retention, all NSAIDs carry with
them the consequent risk of increased fluid retention.14
Additionally, the inhibition of vasodilating prostaglandins and the production
of vasoconstricting factors, namely endothelin-1, can contribute to the
induction of hypertension in a normotensive and/or controlled hypertensive
patient.14
In a comparison of celecoxib
with diclofenac conducted in 287 patients with arthritis, cardiovascular and
renal side effects were seen in 79 patients (27.8%), with hypertension being
the most common (16.6%).14 There was no statistical difference in
the incidence of hypertension between the traditional NSAID and COX-2 groups.
This initiation of hypertension by NSAIDs is especially important in the
discussion of COX-2 safety in light of the fact that hypertensive status is a
key risk factor in the progression of virtually all cardiovascular diseases
including stroke, myocardial infarction, and congestive heart failure.15
A recent meta-analysis of
COX-2 inhibitors and their effects on blood pressure was published.16
Data were collected in 45,451 patients from 19 clinical trials. Interestingly,
there appeared to be a somewhat greater blood pressure elevation with
COX-2 inhibitors compared with placebo and nonselective NSAIDs (e.g.,
ibuprofen and diclofenac). Rofecoxib appeared to confer a greater
risk of developing clinically important elevations in both systolic and
diastolic pressures in comparison to celecoxib. However, rofecoxib
was voluntarily pulled from the market in 2004 due to concerns about increased
risk of heart attack and stroke.17
Because of the widespread
availability of NSAIDs without a prescription, many patients with hypertension
may be at risk for aggravated blood pressure effects caused by these drugs.
Pharmacists should take a careful medication history and specifically inquire
about OTC use of NSAIDs. Patients with hypertension should be more closely
monitored for blood pressure elevations when using NSAIDs. Patients should be
counseled that this adverse effect tends to be dose related, but it is not
always predictable. The adverse effect of all NSAIDs and COX-2 inhibitors on
blood pressure may have the most clinical significance in the
elderly, in whom the prevalence of arthritis, hypertension, and
NSAID use is high.18
Corticosteroids
All corticosteroid
drugs, including prednisone, can cause sodium retention, resulting in
dose-related fluid retention.19 Corticosteroids with strong
mineralocorticoid effects, such as fludrocortisone and hydrocortisone, produce
the greatest amount of fluid retention. However, some corticosteroids that
lack significant mineralocorticoid activity (e.g., dexamethasone,
triamcinolone, betamethasone) may produce minor fluid retention.20
Corticosteroid-induced fluid retention can be severe enough to cause
hypertension, and patients with preexisting hypertension may develop a
worsening of blood pressure control when these drugs are initiated. The
principal mechanism of corticosteroid-induced hypertension is the
overstimulation of the mineralocorticoid receptor, resulting in sodium
retention in the kidney. This results in volume expansion and a subsequent
increase in blood pressure. Corticosteroid-induced hypertension may respond to
diuretic therapy.21 The smallest effective dose and shortest
duration of steroid therapy should be used in order to decrease the
development of this adverse effect.
Fludrocortisone causes
significant blood pressure increases and, thus, is useful in treating patients
with postural hypotension. In a study of 64 elderly patients receiving an
average dose of 75 mcg of fludrocortisone for approximately five months, four
patients had to withdraw because of drug-induced hypertension.22
The study investigators concluded that fludrocortisone therapy was poorly
tolerated in elderly patients, even at low doses.
Caffeine
The effects of caffeine on blood
pressure control are not well defined. A meta-analysis of randomized
controlled trials analyzing the effect of either coffee or caffeine alone on
blood pressure levels was recently published.23 A total of 16
studies with randomized, controlled designs were selected for review,
representing 1,010 subjects. An increase of 2.04 mmHg in systolic blood
pressure and of 0.73 mmHg in dia stolic blood pressure was found after
pooling these trials. When the coffee and caffeine trials were analyzed
separately, the blood pressure elevations induced were larger with caffeine
(410 mg/day) than with coffee (725 mL/day). The effects of coffee and caffeine
on heart rate were not significant.
Estrogens and Progestins
Chronic use of oral contraceptives
may slightly raise blood pressure in certain women and may have other adverse
effects on cardiovascular risk. Early epidemiologic studies using high-dose
estrogen found mean elevations in blood pressure of 3 to 6 mmHg systolic and 2
to 5 mmHg diastolic, with approximately 5% of women developing new
hypertension.24 This was more likely to occur in patients who had
previously developed hypertension during a pregnancy or in those with a family
history of hypertension. Although the rise in blood pressure is usually mild,
malignant hypertension can occur.25 The main concern with an oral
contraceptive–induced rise in blood pressure is the development of persistent
hypertension and subsequent premature cardiovascular disease, especially in
women who smoke. Cessation of therapy typically leads to a return to baseline
blood pressure within two to 12 months, but proteinuria may persist.25,26
The mechanisms responsible for
the hypertensive effect of oral contraceptives are poorly understood. The
renin-angiotensin system may be involved, since estrogen stimulates the
hepatic production of the renin substrate angiotensinogen.27 Both
estrogen and progesterone appear to contribute in a dose-dependent fashion.
The often-quoted 5% incidence of hypertension associated with estrogen is
derived from studies of high-dose therapy in which the estrogen dose was at
least 50 mcg and the progestin dose was 1 to 4 mg.24 However,
current preparations contain as little as 20% of the amount of estrogen and
progestin used in previous preparations. A report from the Nurses' Health
Study prospectively evaluated almost 70,000 female nurses, aged 25 to 42 years.28
After adjustment for age, weight, smoking, family history, and other risk
factors, the relative risk of hypertension in the nurses compared to women who
never used oral contraceptives was 1.8 for current users and 1.2 for previous
users. Overall, only 41.5 cases of hypertension per 10,000 person-years could
be attributed to oral contraceptive use, and this number rapidly declined with
cessation of therapy. In a meta-analysis of 14 studies published between 1980
and 2003, the relative risk of stroke and heart attack increased two-fold in
current users of oral contraceptives (<50 mcg of ethinyl estradiol daily).29
Postmenopausal estrogen
replacement therapy (ERT), or hormone replacement therapy (HRT) when combined
with progestin, consists of much lower estrogen doses than those in oral
contraceptives. ERT and HRT appear to have a neutral effect on blood pressure
as illustrated by the following observations from two large randomized trials.
The Women's Health Initiative (WHI) is the largest (N = 16,000) randomized,
placebo-controlled trial that has evaluated the effect of estrogen-progestin
replacement on outcomes in postmenopausal women.30 At 5.2 years,
HRT produced only a small increase (1.5 mmHg) in systolic pressure compared to
placebo. Similar findings were noted in the PEPI trial in which ERT, with or
without progestins, did not affect blood pressure at three years.31
Additional studies involving
fewer women have found a reduction of ambulatory blood pressure and a greater
decline of nocturnal pressure in ERT users.32,33 It is possible
that HRT may slow the rise in systolic pressure over a longer period of
treatment.34 However, because of the significant increases in
coronary, stroke, and venous thromboembolic risk demonstrated in the WHI, HRT
is no longer recommended for cardiovascular protection.35
Dietary Supplements
Ginseng is
generally recognized as safe and has been associated with few serious side
effects. Because it can have a mild stimulant effect, use with other
stimulants in patients with cardiovascular disease should be cautioned. A type
of ginseng abuse syndrome, characterized by diarrhea, hypertension,
nervousness, dermatologic eruptions, and insomnia, has been described.36
This syndrome may be exhibited after single high doses or prolonged periods of
use. Other supplements that may increase arterial pressure include natural
licorice and yohimbine.37 Generally, all patients with hypertension
should discuss use of dietary supplements with their pharmacist or physician
beforehand. The effects of most supplements on blood pressure have not been
adequately characterized.
Serotonin-Norepinephrine
Reuptake Inhibitors
Venlafaxine:
Venlafaxine is a serotonin-norepinephrine reuptake inhibitor (SNRI) used in
the treatment of depression and anxiety disorders. The likely mechanism of
venlafaxine-induced hypertension is the increase in levels of norepinephrine
and the subsequent potentiation of noradrenergic neurotransmission.38
The extended-release formulation of venlafaxine increases blood pressure in
approximately 3% of patients when normal doses (75-150 mg) are used.38
The majority of these blood pressure elevations, however, were considered
minor. Doses >=300 mg of extended-release venlafaxine demonstrated
clinically significant elevations in 13% of patients, with the majority of
blood pressure increases between 10 and 15 mmHg.39 However, it is
important to note that dosing 300 mg or more is not common, and the risk of
venlafaxine-induced hypertension will usually not warrant the discontinuation
of this drug.40
Sibutramine:
The clinical significance
of sibutramine-induced hypertension is not well defined. Sibutramine is an
SNRI and is chemically similar to amphetamine. Sibutramine's likely mechanism
of blood pressure elevation in both normotensive and hypertensive patients is
the elevated amount of norepinephrine present in the body.41 A
clinical trial evaluating the adverse reactions induced by sibutramine
demonstrated a mean elevation of systolic and diastolic blood pressures of 2
mmHg in previously normotensive patients receiving 10 to 15 mg sibutramine
daily. Interestingly, an elevation of 7 mmHg was demonstrated in hypertensive
patients receiving similar doses.42 Other trials have demonstrated
similar findings.43,44 Patients with established hypertension
receiving sibutramine experienced significantly higher elevations in blood
pressure than patients who had normal blood pressure before medication
initiation. Sibutramine treatment should probably be limited to patients who
do not have cardiovascular disease, including hypertension, functional
abnormalities, and coronary artery disease.
Immunosuppressants
Cyclosporine:
The adverse effect of
cyclosporine on blood pressure is well known.45 The exact mechanism
of cyclosporine-induced hypertension is uncertain, but several hypotheses have
been proposed, including increased prostaglandin synthesis and decreased
water, sodium, and potassium excretion.46,47 Up to 50% of renal
transplant patients receiving cyclosporine treatment have reported elevated
blood pressure, and most of these cases required treatment for hypertension.48
Because of the adverse effects of cyclosporine withdrawal in transplant
patients and in patients with autoimmune disease, cyclosporine is rarely
discontinued for elevated hypertension. Treatment of cyclosporine-induced
hypertension may be pharmacologic, consisting possibly of calcium channel
blockers, diuretics, beta-blockers, or ACE inhibitors, or nonpharmacologic,
consisting of reduced sodium intake.45 In 1999, a consensus
statement was released, stating that if systolic blood pressure rose above 140
mmHg or diastolic pressure rose above 90 mmHg on two consecutive occasions,
then the cyclosporine dose should be decreased by 25%.48 Blood
pressure should be monitored every two weeks for the first three months of
cyclosporine therapy in order to monitor for any changes.
Tacrolimus:
In patients with severe, treatment-refractory cyclosporine-induced
hypertension, switching to tacrolimus may be an option. Tacrolimus, like
cyclosporine, has been shown to have a significant effect on blood pressure.
However, the incidence of tacrolimus-induced hypertension (35%) is less than
that of cyclosporine (50%).49 The mechanism of tacrolimus-induced
hypertension is postulated to be similar to cyclosporine's, as previously
discussed.50 Modifications similar to those listed for
cyclosporine-induced hypertension, whether pharmacologic or nonpharmacologic,
may be required to treat the blood pressure elevations associated with
tacrolimus therapy.51 Careful blood pressure monitoring is
warranted during therapy with either tacrolimus or cyclosporine.
Summary
Pharmacists should
maintain an awareness of the major drug classes that may increase blood
pressure and/or interfere with effective blood pressure control. Examples
include sympathomimetics, NSAIDs, estrogens, corticosteroids, cyclosporine,
and some natural products (e.g., ginseng). Pharmacists should screen for
medications that raise blood pressure and should provide feedback to patients
and medical providers to decrease this potential cause of secondary
hypertension. Generally, all patients with hypertension should be monitored
more closely anytime additional medications are prescribed, especially when
drugs known to raise blood pressure are added.
REFERENCES
1. Cantu C, Arauz
A, Murillo-Bonilla LM, et al. Stroke associated with sympathomimetics
contained in over-the-counter cough and cold drugs. Stroke. 2003;34:1667-1672.
2. Kernan W, Viscoli C,
Brass L, et al. Phenylpropanolamine and the risk of hemorrhagic stroke. N
Engl J Med. 2000;343:1826-1832.
3. Fleming GA. The FDA,
regulation, and the risk of stroke. N Engl J Med. 2000;343:1886-1887.
4. Mersfelder TL.
Phenylpropanolamine and stroke: the study, the FDA ruling, the implications. Cleveland
Clin J Med. 2001;68:213-219.
5. Salerno SM, Jackson
JL, Berbano EP. Effect of oral pseudoephedrine on blood pressure and heart
rate: a meta-analysis. Arch Intern Med. 2005;165:1686-1694.
6. Coates ML, Rembold
CM, Farr BM. Does pseudoephedrine increase blood pressure in patients with
controlled hypertension? J Fam Pract. 1995;40:22-26.
7. Bradley JG.
Nonprescription drugs and hypertension. Which ones affect blood pressure? Postgrad
Med.1991;89:195-197,201-202.
8. 2006 safety alerts
for drugs, biologics, medical devices, and dietary supplements. Dexedrine
(dextroamphetamine sulfate). FDA. August 21, 2006.
www.fda.gov/medwatch/safety/2006/safety06.htm#Dexedrine. Accessed May 13, 2008.
9. Samuels JA, Franco
K, Wan F, Sorof JM. Effect of stimulants on 24-h ambulatory blood pressure in
children with ADHD: a double-blind, randomized, cross-over trial. Pediatr
Nephrol. 2006;21:92-95.
10. Howard PA,
Delafontaine P. Nonsteroidal anti-inflammatory drugs and cardiovascular risk. J
Am Coll Cardiol. 2004;43:519-525.
11. Armstrong EP,
Malone DC. The impact of nonsteroidal anti-inflammatory drugs on blood
pressure, with an emphasis on newer agents. Clin Ther. 2003;25:1-18.
12. Schnitzer TJ.
Cyclooxygenase-2-specific inhibitors: are they safe? Am J Med.
2001;110:46S-49S.
13. DeMaria AN, Weir
MR. Coxibs--beyond the GI tract: renal and cardiovascular issues. J
Pain Symptom Manage. 2003;25(suppl 2):S41-S49.
14. Stollberger C,
Finsterer J. Side effects of conventional nonsteroidal anti-inflammatory drugs
and celecoxib: more similarities than differences. South Med J.
2004;97:209.
15. Fitzgerald GA.
Coxibs and cardiovascular disease. N Eng J Med. 2004;351:1709-1711.
16. Aw TJ, Haas SJ,
Liew D, Krum H. Meta-analysis of COX-2 inhibitors and their effects on blood
pressure. Arch Intern Med. 2005;165:490-496.
17. Merck announces
voluntary worldwide withdrawal of VIOXX. September 30, 2004.
www.merck.com/newsroom/vioxx/pdf/vioxx_press_release_final.pdf. Accessed May
13, 2008.
18. de Leeuw PW.
Drug-induced hypertension. Recognition and management in older patients. Drugs
Aging. 1997;11:178-185.
19. Clyburn EB, DiPette
DJ. Hypertension induced by drugs and other substances. Semin Nephrol.
1995;15:72-86.
20. Hari P, Bagga A,
Mantan M. Short term efficacy of intravenous dexamethasone and
methylprednisolone therapy in steroid resistant nephrotic syndrome. Indian
Pediatr. 2004;41:993-1000.
21. Ferrari P. Cortisol
and the renal handling of electrolytes: role in glucocorticoid-induced
hypertension and bone disease. Best Pract Res Clin Endocrinol Metab.
2003;17:575-589.
22. Hussain RM,
McIntosh SJ, Lawson J, et al. Fludrocortisone in the treatment of hypotensive
disorders in the elderly. Heart. 1996;76:507-509.
23. Noordzij M,
Uiterwaal CS, Arends LR, et al. Blood pressure response to chronic intake of
coffee and caffeine: a meta-analysis of randomized controlled trials. J
Hypertens. 2005;23:921-928.
24. Woods JW. Oral
contraceptives and hypertension. Hypertension. 1988;11:II11-II15.
25. Lim KG, Isles CG,
Hodsman GP. Malignant hypertension in women of childbearing age and its
relation to the contraceptive pill. Br Med J (Clin Res Ed).
1987;294:1057-1059.
26. Ribstein J, Halimi
JM, du Cailar G, Mimran A. Renal characteristics and effect of angiotensin
suppression in oral contraceptive users. Hypertension. 1999;33:90-95.
27. Goldhaber SZ,
Hennekens CH, Spark RF, et al. Plasma renin substrate, renin activity, and
aldosterone levels in a sample of oral contraceptive users from a community
survey. Am Heart J. 1984;107:119-122.
28. Chasan-Taber L,
Willett WC, Manson JE, et al. Prospective study of oral contraceptives and
hypertension among women in the United States. Circulation.
1996;94:483-489.
29. Baillargeon JP,
McClish DK, Essah PA, Nestler JE. Association between the current use of
low-dose oral contraceptives and cardiovascular arterial disease: a
meta-analysis. J Clin Endocrinol Metab. 2005;90:3863-3870.
30. Rossouw JE,
Anderson GL, Prentice RL, et al. Risks and benefits of estrogen plus progestin
in healthy postmenopausal women: principal results from the Women's Health
Initiative randomized controlled trial. JAMA. 2002;288:321-333.
31. Effects of estrogen
or estrogen/progestin regimens on heart disease risk factors in postmenopausal
women. The Postmenopausal Estrogen/Progestin Interventions (PEPI) Trial. JAMA.
1995;273:199-208.
32. Butkevich A,
Abraham C, Phillips RA. Hormone replacement therapy and 24-hour blood pressure
profile in postmenopausal women. Am J Hypertens. 2000;13:1039-1041.
33. Cagnacci A, Rovati
L, Zanni A, et al. Physiological doses of estradiol decrease nocturnal blood
pressure in normotensive postmenopausal women. Am J Physiol.1999;276:H1355-H1360.
34. Scuteri A, Bos AJ,
Brant LJ, et al. Hormone replacement therapy and longitudinal changes in blood
pressure in postmenopausal women. Ann Intern Med. 2001;135:229-238.
35. Bath PM, Gray LJ.
Association between hormone replacement therapy and subsequent stroke: a
meta-analysis. BMJ. 2005;330:342.
36. Chen KJ. The effect
and abuse syndrome of ginseng. J Trad Chin Med. 1981;1:69-72.
37. Pittler MH, Schmidt
K, Ernst E. Adverse events of herbal food supplements for body weight
reduction: systematic review. Obes Rev. 2005;6:93-111.
38. Thase ME. Effects
of venlafaxine on blood pressure: a meta-analysis of original data from 3744
depressed patients. J Clin Psychiatry. 1998;59:502-508.
39. Effexor XR
(venlafaxine HCl) package insert. Philadelphia, PA: Wyeth Pharmaceuticals;
February 2008.
40. Venlafaxine: a new
dimension in antidepressant pharmacotherapy. J Clin Psychiatr.
1993;54:119-126.
41. Lean ME.
Sibutramine: a review of clinical efficacy. Int J Obes Relat Metab Disord.
1997;21(suppl 1):S30-S36.
42. Weintraub M, Rubio
A, Golik A, et al. Sibutramine in weight control: a dose-ranging, efficacy
study. Clin Pharmacol Ther. 1991;50:330-337.
43. King DJ, Devaney N.
Clinical pharmacology of sibutramine hydrochloride, a new antidepressant, in
healthy volunteers. Br J Clin Pharmacol. 1988;26:607-611.
44. Perrio MJ, Wilton
LV, Shakir SA. The safety profiles of orlistat and sibutramine: results of
prescription-event monitoring studies in England. Obesity. 2007;15:2712-2722.
45. Vercauteren SB,
Bosmans JL, Elseviers MM, et al. A meta-analysis and morphological review of
cyclosporine-induced nephrotoxicity in auto-immune diseases. Kidney Int.
1998;54:536-545.
46. Kutkuhn B,
Hollenbeck M, Heering P, et al. Development of insulin resistance and elevated
blood pressure during therapy with cyclosporine A. Blood Press.
1997;6:13-17.
47. Charnick SB,
Nedelman JR, Chang CT, et al. Description of blood pressure changes in
patients beginning cyclosporin A therapy. Ther Drug Monit.
1997;19:17-24.
48. Cush JJ, Tugwell P,
Weinblatt M, Yocum D. US consensus guidelines for use of cyclosporin A in
rheumatoid arthritis. J Rheumatol. 1999;26:1176-1186.
49. Pham S, Kormos R,
Hattler B, et al. A prospective trial of tacrolimus in clinical heart
transplantation: intermediate-term results. J Thorac Cardiovasc Surg.
1996;111:764-772.
50. Fung JJ, Todo S,
Jain A, et al. Conversion from cyclosporine to FK 506 in liver allograft
recipients with cyclosporine-related complications. Transplant Proc.
1990;22:6-12.
51. Jain A, Reyes J,
Kashyap R, et al. Liver transplantation under tacrolimus in infants, children,
adults, and seniors: long-term results, survival, and adverse events in 1000
consecutive patients. Transplant Proc.1998;30:1403-1404.
To comment on this article, contact
rdavidson@jobson.com.





