US Pharm.2022;47(7):HS7-HS12.
ABSTRACT: Interstitial lung disease (ILD) encompasses a diverse group of parenchymal lung disorders that are inherently progressive and fatal. Current treatment recommendations for mild-to-moderate impairment in ILD involve a combination of pharmacologic treatment and supportive care. Nintedanib and pirfenidone have emerged as first-line treatment for ILD, as they have been shown to slow or arrest the progression of ILD, which is the main goal of treatment. Nintedanib and pirfenidone are available through specialty pharmacies, but all pharmacists and prescribers should be prepared to appropriately interpret and navigate drug interactions with these agents in any practice setting.
Interstitial lung disease (ILD) encompasses a diverse group of parenchymal lung disorders that involve progressive infiltration of the pulmonary interstitial space and derangement of normal capillary-alveolar anatomy. Some ILDs have known etiologies, including exposure to antigens such as mold and feathers (hypersensitivity pneumonitis), exposure to environmental toxins (asbestosis), and inflammation caused by connective-tissue diseases such as rheumatoid arthritis and systemic sclerosis. Other ILDs—the idiopathic interstitial pneumonias—have no known cause, including the most common form, idiopathic pulmonary fibrosis (IPF).1
Overview of ILD Pharmacotherapy
Management strategies for ILD, which is inherently progressive and fatal, have changed over time. Immunomodulation with steroids, azathioprine, and/or cyclophosphamide was routine in the past and commonly used by practicing pulmonologists, as noted in the 1999 American Thoracic Society consensus statement.2,3 Elevated serum endothelin concentrations and increased expression of endothelin receptors in lung tissue have been observed in patients with IPF. This led to an exploration of the use of endothelin receptor antagonists (ERAs) such as bosentan, ambrisentan, and macitentan as antifibrotic agents in randomized, controlled trials. Studies to date have shown a trend toward benefit with bosentan and trends toward harm with ambrisentan and macitentan. ERAs are not FDA approved for any ILD-related indication, and they should not be used in the management of ILD.1
A 2005 trial of N-acetylcysteine (NAC) added to a regimen of azathioprine and prednisone concluded that this combination improved lung function (specifically, it preserved forced vital capacity [FVC] and diffusion capacity).4 Subsequently, the PANTHER-IPF trial found that triple therapy with prednisone, azathioprine, and NAC increased the risks of hospitalization and death in patients with IPF, and the three-drug arm of the PANTHER-IPF trial was discontinued.2 Assessment of the NAC monotherapy arm continued; however, because there were no significant differences between NAC and placebo patients in rates of decline of FVC, mortality, or acute exacerbations, the use of NAC in IPF was not supported. In 2014, the INPULSIS and ASCEND trials showed promise for reducing the rate of decline in lung function and lessening disease progression with the use of nintedanib and pirfenidone, respectively.5,6 Gastroesophageal reflux disease and microaspiration have been proposed as risk factors for the development and progression of IPF, which is the reasoning behind the current use of antacids in this patient population.
Overview of ILD Treatment Guidelines
Treatment recommendations for mild-to-moderate impairment involve a combination of pharmacotherapy and supportive care. Pharmacologic recommendations include antifibrotic (nintedanib and pirfenidone) and antacid therapies for eligible patients. Nintedanib and pirfenidone have emerged as first-line treatment for ILD, as they have been shown to slow or arrest the progression of ILD, which is the main goal of treatment. All patients should be evaluated for supplemental oxygen requirements, and evaluation for lung transplantation should be strongly considered given ILD’s unpredictable clinical course. There are strong recommendations against the use of triple therapy.2 Recommendations for pulmonary rehabilitation, steroids for acute exacerbations, and referral to palliative care are conditional.
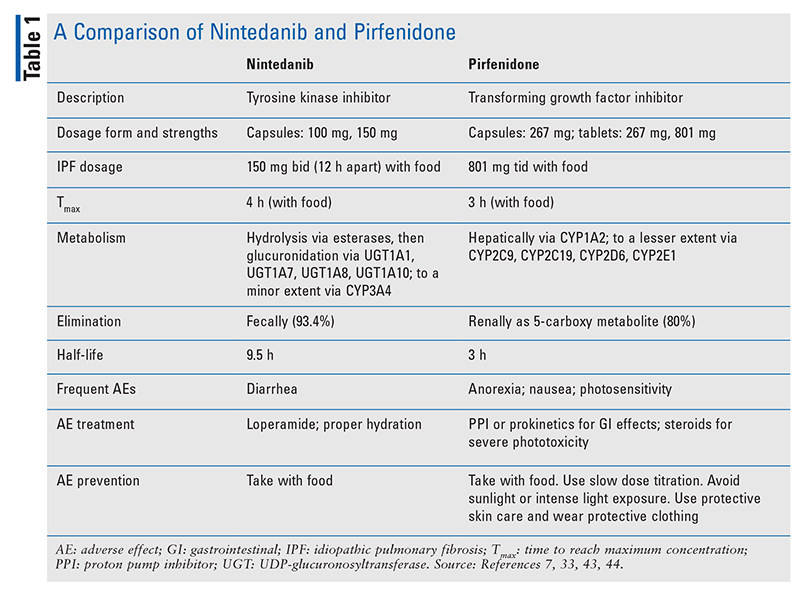
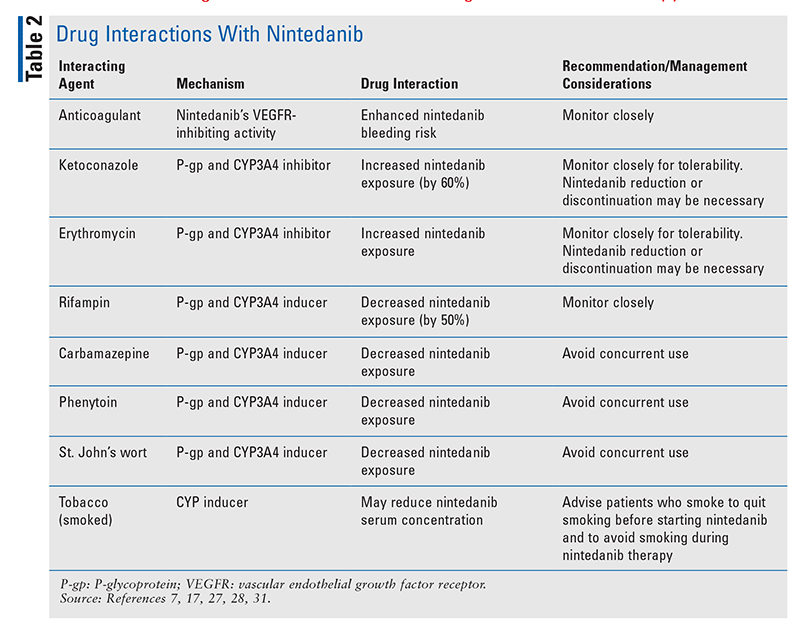
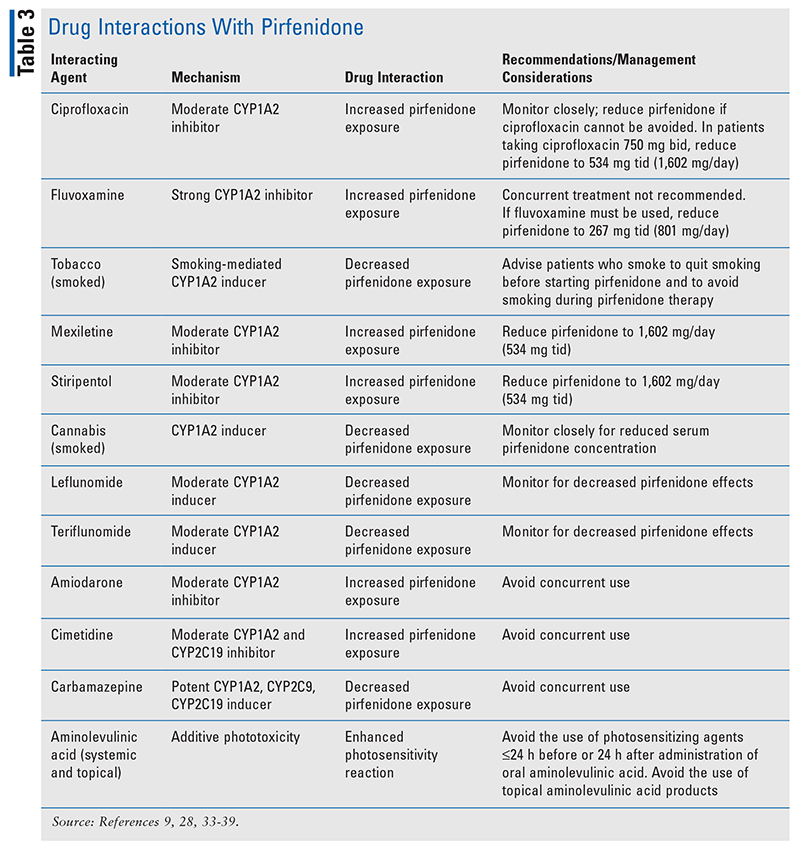
TABLE 1 outlines the pharmacokinetic and pharmacodynamic profiles of pirfenidone and nintedanib. TABLE 2 and TABLE 3 summarize significant drug interactions for nintedanib and pirfenidone, respectively.
Nintedanib
Nintedanib (Ofev) is rapidly hydrolyzed in the liver by esterases (major mechanism) and demethylated by CYP3A4 (minor mechanism) to form inactive metabolites. In general, nintedanib has a low potential for drug interactions with the CYP450 pathway, but coadministration with potent inducers or inhibitors of P-glycoprotein (P-gp) should be monitored closely.7,8 Concurrent use of dual P-gp and CYP3A4 inhibitors (amiodarone, clarithromycin, conivaptan, cyclosporine, dronedarone, erythromycin, fostamatinib, isavuconazonium, itraconazole, ivacaftor, ketoconazole, lapatinib, maribavir, mifepristone, nelfinavir, pacritinib, ranolazine, ritonavir, saquinavir, simeprevir, telaprevir, ticagrelor, tucatinib, verapamil) may increase nintedanib exposure and require nintedanib dose adjustment or treatment interruption.7-26
Concurrent use of P-gp and CYP3A4 inducers may decrease nintedanib exposure. Concurrent use of dual inducers of P-gp and CYP3A4 inducers (carbamazepine, fosphenytoin, mephobarbital, methohexital, pentobarbital, phenytoin, primidone, rifampin, St. John’s wort) should be avoided.7,8,27,28 Advise patients to quit smoking prior to nintedanib initiation, as smoking may induce CYP enzymes and compromise treatment efficacy.7
Nintedanib was found to have no effect on the pharmacokinetics of pirfenidone, suggesting no relevant pharmacokinetic drug-drug interactions with combination use.29 Coadministration of bosentan in healthy subjects did not influence the pharmacokinetics of nintedanib.30
Nintedanib inhibits vascular endothelial growth factor receptors, and patients with a known risk of bleeding should receive nintedanib only if the benefit outweighs the risk. This includes concurrent administration of injectable anticoagulants (heparin, argatroban, bivalirudin, dalteparin, enoxaparin, fondaparinux) and oral agents (warfarin, apixaban, dabigatran, rivaroxaban).7,31
Nintedanib demonstrates pH-dependent aqueous solubility, with increased solubility at pH <3. However, in clinical studies, concurrent administration of proton pump inhibitors or histamine-2 receptor antagonists (drugs that increase gastric pH) did not affect trough concentrations of nintedanib.32
For ILD, the FDA-approved dosage of nintedanib is 150 mg by mouth twice daily (12 hours apart) taken with food. In patients with Child-Pugh class A hepatic impairment, the dose should be decreased to 100 mg. Nintedanib is not recommended in Child-Pugh class B or C impairment, as its safety and efficacy have not been studied in these patients. If the creatinine clearance (CrCl) is 30 mL/minute or greater, dose adjustment is not required. The safety and efficacy of nintedanib for CrCl <30 mL/minute or for end-stage renal disease (ESRD) have not been studied. To monitor nintedanib, liver-function tests (LFTs) should be conducted at baseline, monthly for 3 months, and periodically thereafter. In addition, a pregnancy test should be performed at baseline. The most common adverse effects (AEs) of nintedanib are diarrhea, nausea, abdominal pain, vomiting, and anorexia.7
Pirfenidone
Pirfenidone (Esbriet) is primarily metabolized by CYP1A2 (70%-80%), with minor contributions from other CYP isoenzymes (CYP2C9, CYP2C19, CYP2D6, CYP2E1). Moderate (ciprofloxacin) and strong (fluvoxamine) CYP1A2 inhibitors can increase systemic pirfenidone concentrations and potentially alter the AE profile.33 Concurrent use of pirfenidone with fluvoxamine is not recommended, but if fluvoxamine is the only treatment option, the pirfenidone dose must be reduced (discussed below).33,34 The pirfenidone dose should also be reduced in the case of concomitant ciprofloxacin at a dosage of 750 mg twice daily (see below). Patients taking ciprofloxacin 250 mg or 500 mg twice daily should be monitored closely.33,35 Other moderate CYP1A2 inhibitors that have significant drug interactions with pirfenidone include mexiletine, stiripentol, amiodarone, and cimetidine.9,33,36,37
CYP1A2 inducers (carbamazepine, teriflunomide, leflunomide) can reduce the pirfenidone concentration and lead to a loss of efficacy.28,33,38,39 Concurrent use of strong CYP1A2 inducers should be avoided with pirfenidone.33 Active smoking has resulted in decreased pirfenidone exposure, also due to CYP1A2 induction. Advise patients to stop smoking prior to pirfenidone initiation and to avoid smoking during pirfenidone therapy.33,40
Photosensitivity has been noted to occur with pirfenidone use. It is recommended that other photosensitizing medications be avoided during therapy with pirfenidone. If the combination of pirfenidone and photosensitizers cannot be avoided, it is important to monitor the patient’s skin closely and to counsel the patient to consistently use ultraviolet A/B sunscreen with sun protection factor 50 or higher. Drug classes that commonly increase light sensitivity include antihistamines (diphenhydramine, meclizine, hydroxyzine, doxylamine), oral contraceptives, nonsteroidal anti-inflammatory drugs, phenothiazines, sulfonamides, sulfonylureas, thiazide diuretics, tetracyclines, and tricyclic antidepressants.33,40
For ILD, pirfenidone requires a dose-titration period leading to a maintenance dosage of 801 mg three times daily, taken with food.33 When taken concurrently with fluvoxamine, pirfenidone should be decreased to 267 mg three times daily (801 mg/day).33,34 When taken with concomitant ciprofloxacin 750 mg twice daily, pirfenidone should be decreased to 534 mg three times daily, or 1,602 mg/day.33,35 Caution should be exercised in patients with renal impairment that is mild (CrCl 50-80 mL/minute) or moderate (CrCl 30-50 mL/minute), and pirfenidone should be avoided in patients with ESRD (CrCl <30 mL/minute).33 Specific guidelines on dose adjustments for renal impairment are not available; however, the daily dose may be maintained, reduced, or interrupted as clinically appropriate. Pirfenidone use is not recommended in patients with severe hepatic impairment (Child-Pugh class C). If alanine transaminase and/or aspartate transaminase rises to >3 times the upper limit of normal with hyperbilirubinemia, pirfenidone should be permanently discontinued and the patient should not be rechallenged. Manufacturer recommendations include performing LFTs at baseline and monthly for the first 6 months, every 3 months thereafter, and then as clinically indicated.33,40 Nausea, diarrhea, abdominal pain, and photosensitivity are the most common AEs with pirfenidone.33
The Pharmacist’s Role
ILD treatment is often started in the outpatient clinic setting, but pharmacists have a duty to monitor concurrent prescription and nonprescription medications in every patient encounter regardless of the practice setting. Although pirfenidone and nintedanib are available only from specialty pharmacies, they should be included in medication reconciliation. It is estimated that the annual cost of pirfenidone and nintedanib exceeds $100,000 for each, and the out-of-pocket costs will be challenging for most patients.41,42 Pharmacists can assist patients and prescribers with prior authorization and access to industry-sponsored financial-assistance programs.
Pharmacists should be familiar with the use of pirfenidone and nintedanib for ILD and their respective pharmacologic profiles. These agents differ in their onset of action, half-life, duration of effect, dosage range, and drug-interaction potential. A careful review of drug interactions and subsequent dosage adjustment is essential. Multiple concurrent therapies can impact the efficacy and AE profiles of pirfenidone and nintedanib.
With pirfenidone and nintedanib, smoking should be avoided because it can result in an unpredictable kinetic profile, contributing to disease progression. Patient counseling on smoking avoidance and cessation is a routine component of drug-interaction monitoring.
Conclusion
Current treatment recommendations for mild-to-moderate ILD impairment involve a combination of pharmacologic treatment with antifibrotics (nintedanib or pirfenidone) and antacids plus ongoing supportive care. Nintedanib has the potential for drug interactions with the CYP450 pathway (CYP3A4), and coadministration with potent inducers or inhibitors of P-gp should be monitored closely. Pirfenidone is primarily metabolized by CYP1A2, with minor contributions from other CYP isoenzymes. Smoking can alter the pharmacokinetics of both of these medications, and patients should be instructed to quit smoking before therapy and refrain from smoking during therapy. Nintedanib and pirfenidone are available through specialty pharmacies, but all pharmacists and prescribers should be prepared to accurately interpret and navigate drug interactions with these agents in any practice setting.
REFERENCES
1. Morrow LE, Hilleman D, Malesker MA. Management of patients with fibrosing interstitial lung diseases. Am J Health Syst Pharm. 2022;79(3):129-139.
2. Idiopathic Pulmonary Fibrosis Clinical Research Network; Raghu G, Anstrom KJ, King TE Jr, et al. Prednisone, azathioprine, and N-acetylcysteine for pulmonary fibrosis. N Engl J Med. 2012;366(21):1968-1977.
3. American Thoracic Society. Idiopathic pulmonary fibrosis: diagnosis and treatment. International consensus statement. Am J Respir Crit Care Med. 2000;161(2 Pt 1):646-664.
4. Demedts M, Behr J, Buhl R, et al. High-dose acetylcysteine in idiopathic pulmonary fibrosis. N Engl J Med. 2005;353(21):2229-2242.
5. Richeldi L, du Bois RM, Raghu G, et al. Efficacy and safety of nintedanib in idiopathic pulmonary fibrosis. N Engl J Med. 2014;370(22):2071-2082.
6. King TE Jr, Bradford WZ, Castro-Bernardini S, et al. A phase 3 trial of pirfenidone in patients with idiopathic pulmonary fibrosis. N Engl J Med. 2014;370(22):2083-2092.
7. Ofev (nintedanib) package insert. Ridgefield, CT: Boehringer Ingelheim Pharmaceuticals, Inc; January 2022.
8. Wind S, Schmid U, Freiwald M, et al. Clinical pharmacokinetics and pharmacodynamics of nintedanib. Clin Pharmacokinet. 2019;58(9):1131-1147.
9. Pacerone (amidarone) package insert. Maple Grove, MN: Upsher-Smith Laboratories, LLC; September 2020.
10. Clarithromycin package insert. East Windsor, NJ: Aurobindo Pharma USA, Inc; December 2021
11. Vaprisol (conivaptan) package insert. Deerfield, IL: Baxter Healthcare Corp; September 2017.
12. Neoral (cyclosporine) package insert. East Hanover, NJ: Novartis Pharmaceuticals Corp; June 2021.
13. Multaq (dronedarone) package insert. Bridgewater, NJ: sanofi-aventis U.S. LLC; November 2020.
14. Cresemba (isavuconazonium) package insert. Northbrook, IL: Astellas Pharma US, Inc; February 2022.
15. Sporanox (itraconazole) package insert. Titusville, NJ: Janssen Pharmaceuticals, Inc; December 2019.
16. Kalydeco (ivacaftor) package insert. Boston, MA: Vertex Pharmaceuticals Inc; December 2020.
17. Luedtke D, Marzin K, Jungnik A, et al. Effects of ketoconazole and rifampicin on the pharmacokinetics of nintedanib in healthy subjects. Eur J Drug Metab Pharmacokinet. 2018;43(5):533-541.
18. Livtencity (maribavir) package insert. Lexington, MA: Takeda Pharmaceuticals America, Inc; November 2021.
19. Korlym (mifepristone) package insert. Menlo Park, CA: Corcept Therapeutics Inc; November 2019.
20. Viracept (nelfinavir) package insert. Research Triangle Park, NC: Viiv Healthcare Co; March 2021.
21. Vonjo (pacritinib) package insert. Seattle, WA: CTI BioPharma Corp; February 2022.
22. Ranexa (ranolazine) package insert. Foster City, CA: Gilead Sciences, Inc; October 2019.
23. Norvir (ritonavir) package insert. North Chicago, IL: AbbVie Inc; October 2020.
24. Invirase (saquinavir) package insert. South San Francisco, CA: Genentech, Inc; September 2020.
25. Olysio (simeprevir) package insert. Titusville, NJ: Janssen Therapeutics; November 2017.
26. Incivek (telaprevir) package insert. Cambridge, MA: Vertex Pharmaceuticals Inc; October 2013.
27. Brodie MJ, Mintzer S, Pack AM, et al. Enzyme induction with antiepileptic drugs: cause for concern? Epilepsia. 2013;54(1):11-27.
28. Tegretol (carbamazepine) package insert. East Hanover, NJ: Novartis Pharmaceuticals Corp; March 2020.
29. Richeldi L, Fletcher S, Adamali H, et al. No relevant pharmacokinetic drug-drug interaction between nintedanib and pirfenidone. Eur Respir J. 2019;53(1):1801060.
30. Wind S, Simons G, Bertulis J, Coeck C. Coadministration of bosentan has no effect on the pharmacokinetics on nintedanib [abstract FRI0411]. Ann Rheum Dis. 2017;76(suppl 2):642-643.
31. Grzesk G, Wozniak-Wisniewska A, Blazejewski J, et al. The interactions of nintedanib and oral anticoagulants—molecular mechanisms and clinical implications. Int J Mol Sci. 2021;22(1):282.
32. Nintedanib. AHFS Drug Information Essentials [online database]. Bethesda, MD: American Society of Health-System Pharmacists; 2022.
33. Esbriet (pirfenidone) package insert. South San Francisco, CA: Genentech USA, Inc; February 2022.
34. Luvox (fluvoxamine) package insert. Palo Alto, CA: Jazz Pharmaceuticals, Inc; April 2008.
35. Cipro (ciprofloxacin) package insert. Whippany, NJ: Bayer HealthCare Pharmaceuticals Inc; July 2016.
36. Mexitil (mexiletine) package insert. Ridgefield, CT: Boehringer Ingelheim Pharmaceuticals, Inc; May 2003.
37. Diacomit (stiripentol) package insert. Bedminster, NJ: Biocodex; August 2018.
38. Aubagio (teriflunomide) package insert. Cambridge, MA: Genzyme Corp; April 2021.
39. Arava (leflunomide) package insert. Bridgewater, NJ: sanofi-aventis U.S. LLC; August 2015.
40. Kim ES, Keating GM. Pirfenidone: a review of its use in idiopathic pulmonary fibrosis. Drugs. 2015;75(2):219-230.
41. Lederer DJ, Martinez FJ. Idiopathic pulmonary fibrosis. N Engl J Med. 2018;378(19):1811-1823.
42. Dempsey TM, Payne S, Sangaralingham L, et al. Adoption of the antifibrotic medications pirfenidone and nintedanib for patients with idiopathic pulmonary fibrosis. Ann Am Thorac Soc. 2021;18(7):1121-1128.
43. Serra López-Mantencio JM, Gómez M, Vicente-Rabaneda EF, et al. Pharmacological interactions of nintedanib and pirfenidone in patients with idiopathic pulmonary fibrosis in times of COVID-19 pandemic. Pharmaceuticals (Basel). 2021;14(8):819.
44. Two new drugs for idiopathic pulmonary fibrosis. Med Lett Drugs Ther. 2014;56(1457):123-124.
The content contained in this article is for informational purposes only. The content is not intended to be a substitute for professional advice. Reliance on any information provided in this article is solely at your own risk.
To comment on this article, contact rdavidson@uspharmacist.com.






