US Pharm. 2017;42(6):32-36.
ABSTRACT: In the course of practice, pharmacists are likely to encounter drug-induced skin reactions, which are characterized by different types of skin lesions and can range from mild to life-threatening. There are multiple potential offending agents for each type of skin reaction. Common reaction types include exanthematous reactions, urticaria, allergic contact dermatitis, irritant contact dermatitis, drug hypersensitivity syndrome, fixed drug eruptions, erythema multiforme, serum sickness–like reactions, photosensitivity, skin-pigmentation changes, and pseudoallergic reactions. Pharmacists should be able to identify the common types of drug-induced skin reactions, identify probable offending agents, and make treatment recommendations.
In the course of practice, pharmacists are likely to encounter drug-induced skin reactions, which are characterized by different types of skin lesions and can range from mild to life-threatening. The pharmacist-provided medication therapy management service model in pharmacy practice includes monitoring for adverse drug reactions.1 A comprehensive review of a patient’s prescription, nonprescription, and natural medicines may lead to the detection of adverse effects, which may include a drug-induced skin reaction (DISR). The pharmacist must use problem-solving skills to identify the type of DISR, link the reaction to a specific agent, make appropriate treatment recommendations, and provide patient education.
Assessment and Management
The recognition or identification of a DISR is often a diagnosis of exclusion.2 Possible skin cancers, as well as the presence of a virus, disease, or food-related allergy, should be ruled out. A step-by-step approach should be used to identify a possible DISR:
1. Complete a drug history. Reconciliation of all prescription and nonprescription agents, as well as any natural medicines, should be completed. Develop a written drug-exposure timeline in relation to the adverse reaction, with start and stop dates for all drugs/products.3 Note dates, dosages, and product formulations as soon as possible. Discontinue any suspected drug or product.
2. Physically inspect the affected area, documenting patient signs and symptoms.
3. Determine possible systemic involvement, such as fever, wheezing or difficulty breathing, or skin blistering. Any reaction that involves multiple systemic symptoms or appears to be rapidly worsening should be immediately referred for medical evaluation. Possible DISRs include Stevens-Johnson syndrome and toxic epidermal necrolysis.
4. Identify the drug, classify the type of reaction, make treatment recommendations, and provide patient education to avoid future adverse reactions.
Types of DISRs
Drug reactions may be classified as immune-related reactions and nonimmune-related reactions. Immune-related reactions include exanthematous drug reactions (type IVb, T lymphocyte-eosinophil–mediated; type IVc, T lymphocyte-cytotoxic–mediated); anaphylaxis (type I, IgE [immunoglobulin E]); angioedema (type 1, IgE); urticaria (type 1, IgE); allergic contact dermatitis (type IVa, T lymphocyte-macrophage–mediated); drug hypersensitivity syndrome (DHS; type IVb, T lymphocyte-eosinophil–mediated); fixed drug eruptions; erythema multiforme; and serum sickness–like reactions. Nonimmune-related reactions include drug-induced nail changes, hyperpigmentation and skin-color changes, pseudoallergy, and selective cutaneous reactions. Specific DISRs with images may be accessed on the free website DermNet New Zealand or the VisualDX clinical decision-support software system.4,5
Immune-Related Drug Reactions
Exanthematous Drug Eruptions: Exanthematous drug eruptions, also known as morbilliform or maculopapular drug eruptions, are the most common of all drug-induced reactions.6 This type of reaction, which is delayed, manifests 1 to 2 weeks after drug initiation but can occur up to 1 week after the drug is stopped; it generally resolves 1 to 2 weeks after discontinuation. After drug discontinuation, the reaction sometimes gets worse before it gets better.6 Upon subsequent exposure, the eruption usually reappears in 1 to 3 days. The pruritic eruption generally appears on the trunk or intertriginous areas and then spreads symmetrically to the limbs and neck. Hands, feet, and mucous membranes are usually not affected.2,6 In most cases, the distribution is bilateral and symmetrical. The rash ranges from erythematous pinpoint (measles-like) lesions to lesions of >4 mm that are oval or round without blisters or pustules. The borders are bright red, and the entire lesion maintains a uniform color. If the rash progresses, new lesions may appear and the original lesions may get larger. Mild-to-severe itching is a symptom. A widespread reaction with systemic involvement is known as DHS or a drug reaction with eosinophilia and systemic symptoms (DRESS). Drugs commonly linked to exanthematous drug reactions are listed in TABLE 1.2-14 Treatment for drug-induced reactions is described in TABLE 2.5
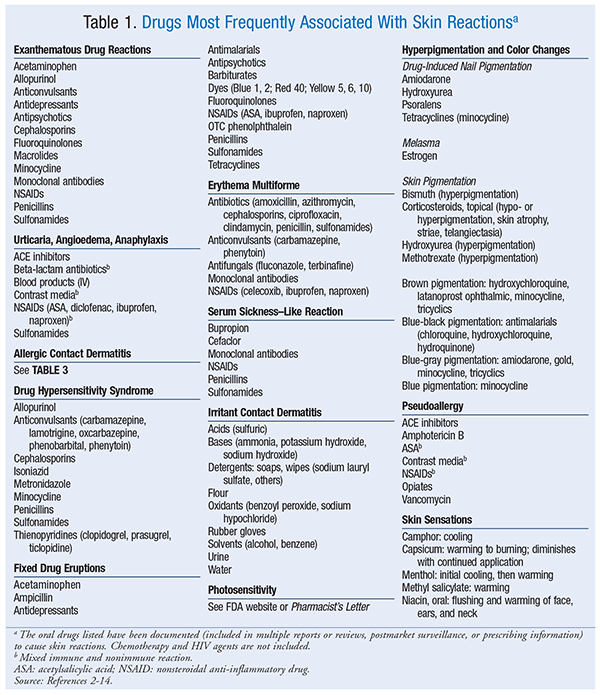
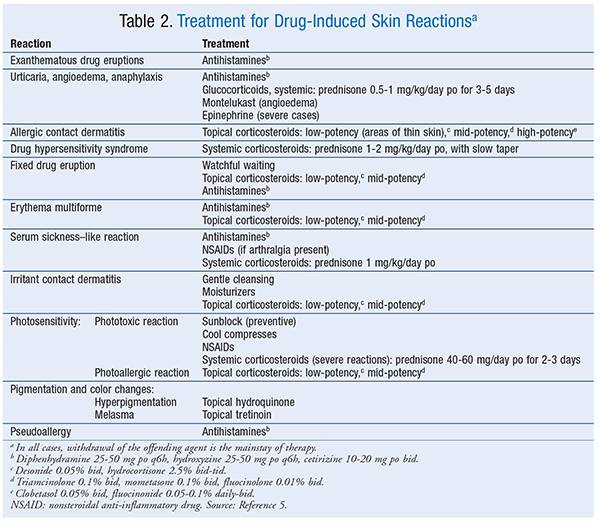
Urticaria, Angioedema, Anaphylaxis: Urticaria, angioedema, and anaphylaxis, which are type 1 IgE–mediated reactions, may occur in combination or individually.2,5 These reactions take place when IgE binds to a drug, triggering the release of inflammatory mediators. Symptoms emerge within minutes to hours of exposure to the offending agent.5,7 The nonimmunologic anaphylactic reaction causes a direct release of histamine (see discussion in Nonimmune-Related Drug Reactions section). Urticaria (i.e., hives) is a rash consisting of pruritic, erythematous wheals.2,5,6 Angioedema refers to the swelling of dermal tissues, particularly in the face and airway.5 Anaphylaxis is a life-threatening response that occurs within minutes of exposure to the sensitizing agent, often in combination with urticaria and angioedema; it is characterized by respiratory distress caused by airway swelling and closing and may be followed by a rapid drop in blood pressure and shock.5,6 Gastrointestinal distress may also occur.2,5,6 Nearly all drugs can cause this type of immune reaction. See TABLE 1 for a list of common causative agents.
Allergic Contact Dermatitis: Allergic contact dermatitis usually occurs within 48 to 72 hours of contact with the allergen; the extent of the reaction depends on the patient’s sensitivity, as well as the specific allergen.10 The initial reaction occurs at the site of contact after exposure to the allergen. Contact dermatitis rash is vesicular and pruritic (at times extreme), with redness at points of contact with the allergen that can spread beyond; it has visible borders and usually leads to erythema and scaling. Acute cases may involve a dramatic flare with erythema, vesicles, and bullae; chronic cases may involve thickened skin with cracks and fissures.
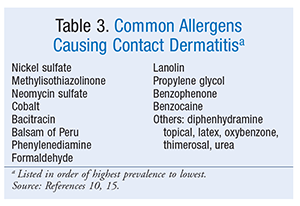
Common agents include urushiol (poison ivy), nickel, oxybenzone, neomycin, and topical benzocaine; slow-reacting agents include neomycin, copper, and nickel.10 If the contact allergen cannot be identified, a patch test is recommended for diagnosis. The most common allergens identified through patch testing appear in TABLE 3; nickel is the most prevalent agent identified.10,15 Nickel is found in garment closures, coins, jewelry, paper clips, razor blades, tools, and foods. Methylisothiazolinone, a preservative, is used in cosmetics, moisturizers, and household products such as liquid laundry detergent, shampoo, and wet wipes. Cobalt is a component of cement. Balsam of Peru (commonly listed as balsam) is found in fragrances, flavorings, and OTC products. Phenylenediamine is a hair dye. Natural medicines associated with contact dermatitis include aloe vera topical, ginkgo, balsam of Peru, propolis, topical tea tree oil, and topical vitamin E.16
DHS: DHS is a serious type IVb reaction believed to be an idiosyncratic or immune-type drug reaction.5,17 In the past, DHS was associated with DRESS, but not all reactions include the eosinophilia component; therefore, DHS is a more proper descriptive term. Findings vary, with a range of symptoms that usually begin 1 to 8 weeks after the initial exposure, may worsen after the drug is stopped, and may be fatal if not promptly treated.2,9,17 DHS is a drug-induced maculopapular eruption accompanied by eosinophilia (at times), fever, facial edema, lymphadenopathy, pharyngitis, oral ulcers, and organ dysfunction (hepatic, renal, or hematologic).9,11,17 The erythematous rash ranges in color from mild pink to deep red, with macules or papules that are arranged symmetrically on the face and then spread down the body.5 Symptoms may persist for 2 to 3 weeks after drug discontinuation.11 Patients with DHS often experience worsening of systemic complications after the initial drug reaction begins to resolve.3 Common drugs associated with DHS are listed in TABLE 1.
Fixed Drug Eruptions: These eruptions are circular erythematous lesions that are painful or pruritic. Lesions appear within 30 minutes to 8 hours of drug exposure and will reappear at the same site upon reexposure. Lesions, which may be solitary or multiple, are most often located on the hands, legs, face, lips, genitalia, and oral mucosa.11 If the rash blisters, further evaluation is required to rule out toxic epidermal necrolysis.6 Lesions are dull red to brown plaques that may have central bullae. Skin hyperpigmentation occurs after the initial lesions subside. Dyes and other inactive ingredients in drugs may trigger an allergic reaction.18 Information on dye content is listed in the package insert. Drugs that are linked to fixed drug eruption appear in TABLE 1.
Erythema Multiforme: Erythema multiforme is believed to be an immune-mediated reaction that can be mistaken for a fixed drug eruption. Less than 10% of erythema multiforme eruptions are linked to a drug.19 Drug-associated erythema multiforme varies in presentation but may be identifiable because lesions appear within 24 to 72 hours after exposure and clear in 2 weeks.19 In most adult cases, the trigger is herpes simplex virus; in children, the most common triggers are penicillins, infections, and viruses. The symmetrical lesions appear on distal limbs and may progress to the middle of the body, appear on mucous membranes, or both. Classic lesions are circular papules <3 cm with a center target. Classic lesions have three different color zones with a middle bulla or crust. Atypical erythema lesions appear as raised “irregular” circular papules with two color regions. In some cases, patients may complain of fever and malaise and may experience organ dysfunction, which can be detected via abnormal laboratory values. See TABLE 1 for drugs linked to erythema multiforme.
Serum Sickness–Like Reaction (SSLR): SSLR does not involve circulating immune complexes or antibodies, as is the case with classic serum sickness. SSLR occurs within 5 to 21 days of drug administration.5 Commonly, the maculopapular or urticarial rash appears on the lower abdominal area or under the arms and spreads to the back, lower trunk, and extremities. If the drug has been administered SC or IM, the rash will begin around the injection site. As with serum sickness, the rash is usually preceded by a prodromal phase consisting of fever, malaise, and lymphadenopathy. Viral infections must be ruled out, as they can mimic SSLR. SSLR is usually self-limiting and resolves within days to weeks after discontinuation of the causative agent, but it can sometimes progress to include upper-airway edema or cause vasculitis. Drugs associated with SSLR are listed in TABLE 1.
Nonimmune-Related Drug Reactions
Irritant Contact Dermatitis: Irritant contact dermatitis is the result of friction; overexposure to elements such as water, heat, or cold; or exposure to tapes, chemicals, solvents, or detergents. In most instances the rash appears at the site of contact with the offending agent, such as the hands or the webs between fingers. An irritant response may be the initial reaction, with a coexisting allergic contact dermatitis appearing later. The rash appears as well-defined erythematous patches that are dry and cracked or appear macerated. Pruritus, burning, and pain are common, and if the itching is not treated, fissures and thickened skin with crusting areas will develop.10,15 The rash will resolve if the offending agent is avoided. The adhesive backings of transdermal patches are linked to irritant contact dermatitis, with signs of erythema and symptoms of itching or burning occurring in up to 50% of patients.20 Patch discontinuation may be avoided if the patch is gently removed from the skin and the site of application is rotated. TABLE 1 lists agents associated with irritant contact dermatitis.
Photosensitivity: The most common photosensitivity reaction is phototoxicity, which appears as an exaggerated sunburn on sun-exposed skin. Drugs that are phototoxic absorb ultraviolet A (UVA) radiation and undergo a chemical change to cause skin reactions.21 A photoallergic reaction, which results when UVA radiation triggers an antigenic reaction, occurs 24 hours after sun exposure. This reaction is a mild-to-severe, pruritic, contact dermatitis–like rash that appears on both sun-exposed and non–sun-exposed skin. Proper use of a sunscreen that protects against UVA radiation will prevent both phototoxic and photoallergic reactions.21 Pharmacist’s Letter and the FDA have published extensive lists of photosensitizing agents.12,22
Pigmentation and Color Changes: Hyperpigmentation results from a rapid increase in the number of melanocytes or an increase in pigment production.23 Drugs that can cause hyperpigmentation include those that produce fixed drug eruptions (TABLE 2). Specific drugs associated with color changes or pigment changes in nails or skin are given in TABLE 1. Melasma, the appearance of symmetrical brown patches on the face—especially the cheeks, upper lip, and forehead—is associated with high levels of estrogen. Melasma associated with pregnancy is known as chloasma. Melasma is more common in women from Asia, South America, and the Middle East.20 Upon discontinuation of estrogen, skin discoloration due to melasma may be permanent.24
Pseudoallergy: A pseudoallergy mimics the presentation of an immune reaction, but it is not truly immune-mediated. The reaction is caused by the release of histamine and ranges from localized hives to life-threatening angioedema, hypotension, and anaphylaxis.5 Pseudoallergic reactions may be as severe as immune-mediated reactions, and their treatment is the same. Drugs that are linked to pseudoallergic reactions are given in TABLE 1. The classic example, red man syndrome, is from vancomycin. A rapid infusion of vancomycin can provoke the direct release, from cutaneous mast cells, of histamine and other mediators that cause itching, flushing, and hives, first around the neck and face and then progressing to the chest and other parts of the body.5 The anticonvulsants phenytoin, phenobarbital, carbamazepine, and lamotrigine are associated with nonimmune hypersensitivity reactions.2
Skin Sensations: Drugs that produce skin sensations are listed in TABLE 1.
Conclusion
Skin reactions may be caused by exposure to certain medications. Patient signs and symptoms help the healthcare professional identify the specific type of DISR. Additionally, the association can be made with use of a medication history, medication reconciliation, and drug-exposure timeline. After identification, the offending agent should be discontinued, treatment recommendations should be made, and patient education should be provided to prevent future adverse reactions.
REFERENCES
1. Medication Therapy Management in Pharmacy Practice: Core Elements of an MTM Service Model Version 2.0. Washington, DC, and Arlington, VA: American Pharmacists Association and National Association of Chain Drug Stores Foundation; March 2008.
2. Law RM, Law DTS. Dermatologic drug reactions and common skin conditions. In: DiPiro JT, Talbert RL, Yee GC, et al, eds. Pharmacotherapy: A Pathophysiologic Approach. 10th ed. New York, NY: McGraw-Hill Education; 2017.
3. Ardern-Jones MR, Friedmann PS. Skin manifestations of drug allergy. Br J Clin Pharmacol. 2011;71:672-683.
4. DermNet New Zealand. www.dermnetnz.org/. Accessed February 25, 2017.
5. VisualDX. www.visualdx.com. Accessed February 25, 2017.
6. Shinkai K, Stern RS, Wintroub BU. Cutaneous drug reactions. In: Kasper DL, Fauci AS, Hauser S, et al, eds. Harrison’s Principles of Internal Medicine. 19th ed. New York, NY: McGraw-Hill Education; 2015.
7. Bircher AJ. Exanthematous (morbilliform) drug eruption. UpToDate. www.uptodate.com/contents/exanthematous-morbilliform-drug-eruption. Accessed February 27, 2017.
8. Bliss SA, Warnock JK. Psychiatric medications: adverse cutaneous drug reactions. Clin Dermatol. 2013;31:101-109.
9. Frew A. General principles of investigating and managing drug allergy. Br J Clin Pharmacol. 2011;71:642-646.
10. Plake KS, Darbishire PL. Contact dermatitis. In: Krinsky DL, Ferreri SP, Hemstreet B, et al, eds. Handbook of Nonprescription Drugs. 18th ed. Washington, DC: American Pharmacists Association; 2015:639-652.
11. Zalewska-Janowska A, Spiewak R, Kowalski ML. Cutaneous manifestation of drug allergy and hypersensitivity. Immunol Allergy Clin North Am. 2017;37:165-181.
12. Drug-induced photosensitivity. Pharm Lett. 2015;31:310807.
13. O’Neal KS, Crosby KM. Skin hyperpigmentation and photo aging. In: Krinsky DL, Ferreri SP, Hemstreet B, et al, eds. Handbook of Nonprescription Drugs. 18th ed. Washington, DC: American Pharmacists Association; 2015:713-724.
14. Olenak JL. Musculoskeletal injuries and disorders. In: Krinsky DL, Ferreri SP, Hemstreet B, et al, eds. Handbook of Nonprescription Drugs. 18th ed. Washington, DC: American Pharmacists Association; 2015:97-112.
15. DeKoven JG, Warshaw EM, Belsito DV, et al. North American Contact Dermatitis Group patch test results 2013-2014. Dermatitis. 2017;28:33-46.
16. Natural Medicines Comprehensive Database. http://naturaldatabase.therapeuticresearch.com/home.aspx?cs=&s=ND. Accessed February 25, 2017.
17. Common skin disorders. In: Herrier RN, Apgar DA, Boyce RW, Foster SL, eds. Patient Assessment in Pharmacy. New York, NY: McGraw-Hill Education; 2015.
18. Management of common skin diseases. Pharm Lett. 2007;23:231011.
19. Sokumbi O, Wetter DA. Clinical features, diagnosis, and treatment of erythema multiforme: a review for the practicing dermatologist. Int J Dermatol. 2012;51:889-902.
20. Ale I, Lachapelle JM, Maibach HI. Skin tolerability associated with transdermal drug delivery systems: an overview. Adv Ther. 2009;26:920-935.
21. Crosby KM, O’Neal KS. Prevention of sun induced disorders. In: Krinsky DL, Ferreri SP, Hemstreet B, et al, eds. Handbook of Nonprescription Drugs. 18th ed. Washington, DC: American Pharmacists Association; 2015:699-712.
22. FDA. The sun and your medicine. www.fda.gov/Drugs/ResourcesForYou/SpecialFeatures/ucm464195.htm. Accessed February 25, 2017.
23. Bolognia JL, Braverman IM. Skin manifestations of internal disease. In: Kasper DL, Fauci AS, Hauser S, et al, eds. Harrison’s Principles of Internal Medicine. 19th ed. New York, NY: McGraw-Hill Education; 2015.
24. Craft N, Fox LP, Goldsmith LA, et al, eds. VisualDX: Essential Adult Dermatology [clinical decision-support software system]. www.visualdx.com/essential-dermatology/adult. Accessed May 2, 2017.
To comment on this article, contact rdavidson@uspharmacist.com.





