US Pharm. 2024;49(11):HS-12-HS-16.
ABSTRACT: In treating recurrent Clostridioides difficile infections, exploring alternatives to antibiotics is essential due to the risk of further disrupting gut microbiota. These alternatives include traditional fecal microbiota transplantation (FMT) and newer types of FMT in the form of live biotherapeutic products (LBPs). While traditional FMT relies on nonstandardized donor stool samples, LBPs provide a more uniform and consistent approach to microbiota transplantation. The FDA has approved two LBPs, Rebyota and Vowst, as pharmaceutical-grade options, with additional products in development. Pharmacists play a crucial role in educating patients about the origins and therapeutic roles of these products.
Clostridioides difficile is a spore-forming bacterium that is primarily responsible for causing infectious diarrhea, especially in patients who were recently treated with antibiotics such as fluoroquinolones, certain cephalosporins, and clindamycin. The administration of these and other antibiotics disrupts the gut microbiota, enabling C diff to colonize and proliferate in the intestines. C diff infection (CDI) is the most common healthcare-associated infection, contributing to prolonged hospital stays and higher mortality rates.1
Historically, metronidazole and vancomycin, both relatively broad–spectrum antibiotics, were the standard treatments for CDI. These drugs are linked to disappointingly high rates of CDI recurrence, however, likely because they disrupt the gut microbiota during treatment.2 Fidaxomicin, a narrower-spectrum antibiotic, has notably lower recurrence rates and an improved safety profile compared with vancomycin.1,2 The adoption of this newer option has been limited due to its high cost, however.1
Recurring Episodes of CDI
CDI is notoriously challenging to treat, with recurrent infections posing significant hurdles that demand innovative approaches. As a major public health concern, recurring C diff infections (rCDIs) often necessitate fecal microbiota transplantation (FMT) after two or more recurrent episodes. FMT, which involves restoring the gut microbiome using either donor stool samples or cultured cell banks, has shown promising results across various applications.
After an initial episode of CDI, patients face up to a 25% risk of rCDI, with those experiencing multiple recurrences having up to a 75% chance of subsequent recurrence.2 The Infectious Diseases Society of America (IDSA) and the Society for Healthcare Epidemiology of America (SHEA) define rCDI as a recurrence within 8 weeks of resolving a previous episode, even after successful treatment.1 Their guidelines recommend considering FMT as an alternative therapy for patients with rCDI after completing antibiotic treatment.3 The IDSA suggests that FMT should be offered only after appropriate antibiotic treatments for at least two recurrences (i.e., three CDI episodes).3
What Is FMT?
FMT is a procedure that introduces healthy donor stool into a patient’s intestines to restore his or her gut microbiota to a healthy state.4 It involves transferring minimally processed microorganisms from a donor to a recipient, with fecal material specifically used in this process. Fecal samples are typically diluted with sterile liquids and administered shortly after preparation.5 They are usually delivered rectally via an enema during colonoscopy or through nasogastric or nasoduodenal tubes.5,6 Oral FMT via extended-release capsules has shown efficacy that is comparable with other delivery methods and is available from stool banks and research centers.6 This option is suitable for patients who do not need a colonoscopy and can tolerate oral administration, although younger children are generally not candidates due to the size and number of capsules.6
The microbial composition of each FMT intervention can vary significantly due to differences in donor stool and preparation methods used by various facilities.5 While potential FMT donors must undergo thorough screening and selection, there are limited data on how donor characteristics affect FMT outcomes.5,6 Current recommendations for donor selection are based on expert opinion rather than solid evidence.6 This lack of standardization and variability in screening practices present challenges for both regulators and clinicians, making FMT preparation more of an art than a science.6
FMT is highly effective in preventing recurrence of CDI because it directly repopulates the intestinal microbiota rather than merely suppressing C diff growth, thereby restoring microbiota diversity and function.7 FMT therapy has achieved ~92% efficacy in treating rCDI and has proven to be superior to fidaxomicin and standard-dose vancomycin monotherapies when used after a short course of vancomycin.1,8 Studies indicate that FMT significantly alters the recipient’s gut microbiome, especially the abundance of bacteroidetes, to resemble that of the healthy donor.5
Live Biotherapeutic Products
Although traditional FMT methods are considered relatively safe, concerns persist regarding the acquisition of donor fecal material and its transfer to patients, particularly those who are immunocompromised. Despite a stringent donor-selection process, the FDA has issued safety alerts about FMT due to the risk of transmitting pathogenic bacterial strains.2,5,9 In 2019, the FDA issued a safety alert after two immunocompromised adults developed invasive Escherichia coli infections following FMT, and norovirus gastroenteritis has been reported in FMT recipients despite asymptomatic donors.5,9 Aspiration is a risk when delivering FMT through an endoscope, with one instance leading to the death of an 80-year-old patient with rCDI.6 Additionally, the COVID-19 pandemic underscored the relevance of pathogen transmission risks from donor to recipient, imposing restrictions and complicating stool donor recruitment.2,11,12 Given these concerns with FMT, the need arose to formulate uniform FMT products with enhanced safety profiles while minimizing inconsistencies.
According to FDA definitions, live biotherapeutic products (LBPs) are nonvaccine biological agents containing live organisms, such as bacteria, that are used for preventing, treating, or curing diseases or conditions in humans.2,5 LBPs are given as a targeted form of FMT therapy after the completion of standard antibiotic treatment for rCDI. LBPs differ from traditional FMT, as they are classified by the FDA as biological drug products, marking a regulatory shift for these therapies, as shown in TABLE 1.2,5 Since FMT involves marginally processed donor stool, the FDA classifies traditional FMT as an investigational drug agent.2 In contrast, LBPs are required to be produced following Good Manufacturing Practice guidelines and must be studied in clinical trials to obtain FDA approval, similar to pharmaceutical drugs.2,5,11
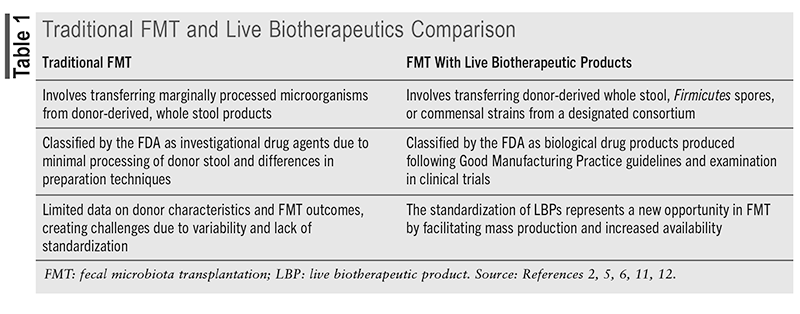
Current LBPs can be categorized by their microbial sources, with one type being derived from human donors. Healthy stool donors participate in consistent screening for 29 different pathogens to reduce the risk of contagion, as mandated by the FDA.5,12 These products provide greater standardization in manufacturing, often incorporating additional filtration and treatment to refine the bacterial selection in whole stool donations. Akin to traditional FMT, however, the quantity and diversity of microbial specimens can differ between batches of donor-derived LBPs. The FDA acknowledges this variability and expects donor stool samples to generally reflect the microbial composition typical of the broader population.
In a second method, a consortium of multiple microbes is engineered as a drug, utilizing cell-line isolation to deliver a specific selection of beneficial bacterial cultures.13 The standardization of LBPs represents a major leap forward in FMT, shifting from the complexity of multiple investigational new drug applications to the potential for mass production and broader availability.
Commercially Available LBPs
LBPs are recommended for preventing recurrence in patients who have experienced two or more episodes of CDI following tapered treatment with vancomycin or fidaxomicin.5,9 The FDA has approved two LBP products for the prevention of rCDI: Rebyota (fecal microbiota, live-jslm), approved in 2022, and Vowst (fecal microbiota spores, live-brpk), approved in 2023. Vowst is estimated to cost $17,500, while Rebyota costs approximately $9,100, as presented in TABLE 2.1 This expense may be justified, however, when compared with the high cost of hospitalization for rCDI, which ranges from $67,837 to $82,268.10 Rebyota is a cryopreserved and filtered whole stool product containing a diverse range of fecal microbes, including Bacteroides, that is delivered in a rectal solution.2,5,9,12 Stool from qualified donors is routinely screened for multidrug-resistant strains, and each batch of Rebyota is tested to ensure standardization with desired live counts of specific bacterial species.2,5 No bowel preparation is needed before administering Rebyota, and the single-dose procedure should occur 24 to 72 hours after the final dose of CDI antibiotics.9 Rebyota is given by a healthcare provider as a enema using gravity flow, with the patient positioned on his or her left side or in a knee-chest position.5,12 The patient should remain in this position for up to 15 minutes to reduce the chance of cramping, and there are no limitations on restroom use afterward.
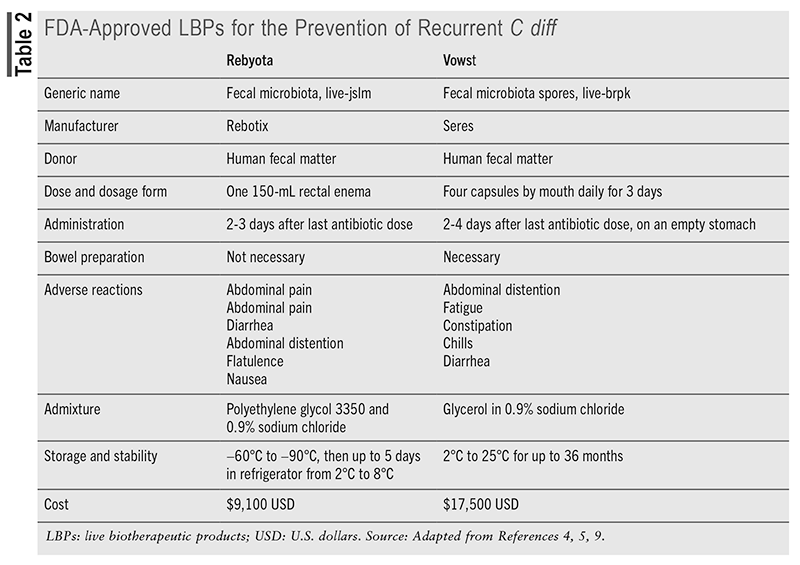
Of the pharmaceutical-grade LBPs, Rebyota most resembles traditional FMT therapies. Rebyota warrants specific handling and requires storage in a frozen state before being transferred to a refrigerator within 5 days of use.5,10 Procurement options include ordering through a specialty distributor or specialty pharmacy and having it administered by a healthcare professional at any care site. For patients facing location constraints, physical limitations, or administrative challenges, the Rebyota @ Home service manages the process, with in-home health nurses coordinating administration.14
Clinical support for Rebyota use comes mainly from four published clinical trials collectively termed the PUNCH studies. Rebyota consistently demonstrated superior effectiveness compared with antibiotic-only treatments for preventing rCDI, with over 90% of successful patients remaining recurrence-free after 6 months.5,9 It is generally well tolerated, with mild gastrointestinal side effects such as abdominal pain (8.9%), diarrhea (7.2%), abdominal distention (3.9%), and flatulence (3.3%) being the most commonly reported.9 Rebyota showed improvements in health-related quality of life, taking into account the drug’s cost and its role in reducing hospitalizations.12
Vowst (fecal microbiota spores, live-brpk) is FDA approved for preventing rCDI and is the first oral LBP, provided in capsules derived from the fecal samples of healthy donors.9,12 Vowst comprises a blend of purified spores from about 50 species within the Firmicutes phylum, which work by restoring gut microbiota and inhibiting the germination, replication, and toxin production of C diff.1,12 Donor stool undergoes an ethanol-based inactivation step to eliminate nonspore organisms, followed by filtration to isolate Firmicutes spores and remove any residuals.9 The spore concentration is then measured to ensure consistency, resulting in a standardized mix of Firmicutes spores that can withstand gastrointestinal acids when administered orally.2,9
Patients should have completed anti–C diff antibiotics and wait 2 to 4 days after the last antibiotic dose before taking the first dose of Vowst.1,12 Additionally, magnesium citrate should be taken the night before beginning Vowst as a bowel cleanse and to neutralize any remaining antibiotics in the gastrointestinal tract.1,9,12 Four capsules should be taken daily for 3 consecutive days (totaling 12 capsules) on an empty stomach before the first meal of the day, with only minimal water allowed during this period.9,12 The prescription capsules are ordered through a Vowst-designated specialty distributor or specialty pharmacy.
Four clinical trials demonstrated the efficacy of Vowst, with treatment-related adverse events primarily being mild to moderate and mainly gastrointestinal. In one study, CDI recurrence within 8 weeks was 12% in the Vowst group, while it was 40% in the placebo group.9,12 Another study found that Vowst administration resulted in microbiome repair and maintained a sustained response for up to 24 weeks after treatment.12 The main benefit was found to be its ability to restore colonization resistance in the gut and enhance metabolic competition against C diff.1 Safety data indicated that the most frequent adverse reactions were abdominal distention (31%), constipation (14.4%), and diarrhea (10.0%), with a hypersensitivity reaction being the only serious adverse event.5,9 As with other LBPs and FMT procedures, Vowst is also subject to warnings about the potential risk of transmitting infectious organisms.
Investigational LBPs
RBX7455, also developed by Rebiotix, is a standardized, lyophilized LBP currently being tested for oral use in patients with rCDI.5,15 This product is produced using doubly encapsulated V-caps and is derived from the same microbes used in the Rebyota enema.15 In addition to its use for rCDI, RBX7455 is being evaluated for its immunomodulatory effects in conditions such as breast cancer, hepatic encephalopathy, and Crohn’s disease.5 Rebiotix intends to have RBX7455 approved as an oral alternative to Rebyota.
VE303 is an investigational LBP consisting of a unique blend of Clostridial species and is administered orally in capsule form.5,9 Unlike other LBPs that use fecal donor material, VE303 is produced from eight different Clostridial strains grown from colonial cell banks, which may enhance consistency and standardization.5,11 It requires no bowel preparation and is taken as 10 capsules daily for 14 days.11 VE303 received Orphan Drug Designation in 2017 and Fast Track Designation in 2023 from the FDA for preventing rCDI.5 Both phase I and phase II trials have met primary endpoints, showing promise for future large-scale studies.1 Additionally, VE303 is under investigation for the treatment of hepatic encephalopathy.5
Other investigational LBPs include CP101 by Finch Therapeutics Group and MET-2 by MaaT Pharma.2,15 CP101 is an oral, single-dose, lyophilized capsule containing a diverse microbiota that has received FDA breakthrough therapy and fast-track designations.1,5 While the specific bacterial components and colony-forming units per dose are not disclosed, Finch Therapeutics holds patents for treatments targeting C diff with a mixture of actinobacteria, proteobacteria, firmicutes, and bacteroidetes.15 MET-2 comprises a proprietary blend of 40 species, purified and lyophilized into capsules for oral use. Initially isolated from healthy donor stool, MET-2 is manufactured independently of the donor to mitigate risks associated with donor health changes.15,16
Role of the Pharmacist
For decades, there were no pharmaceutical-grade FMT options available until the FDA approved Rebyota. Fecal material for FMTs is often sourced from stool banks, such as the nonprofit OpenBiome. These banks collect, screen, and store stool from healthy donors, which physicians then use for patient treatment.17 The shift from traditional FMT to pharmaceutical-grade LBPs offers pharmacists greater opportunities to engage in counseling and patient care. Pharmacists can participate in several stages of LBP delivery, including sourcing, facilitation of health insurance coverage, and patient counseling. In addition, since LBPs contain active bacteria, their use raises concerns compared to traditional drugs, accentuating the need for further research to refine adjunctive therapies and improve outcomes through pharmacovigilance.1 On the whole, effective treatment for rCDI requires collaboration among infectious disease physicians, pharmacists, and other specialists to ensure comprehensive and coordinated patient care.18
REFERENCES
1. Jain N, Umar TP, Fahner AF, Gibietis V. Advancing therapeutics for recurrent Clostridioides difficile infections: an overview of vowst’s FDA approval and implications. Gut Microbes. 2023;15(1):2232137.
2. Lavoie T, Appaneal HJ, LaPlante KL. Advancements in novel live biotherapeutic products for Clostridioides difficile infection prevention. Clin Infect Dis. 2023;77(Suppl 6):s447-s454.
3. Mada PK, Alam MU. Clostridioides difficile infection. In: StatPearls. StatPearls Publishing; 2024. www.ncbi.nlm.nih.gov/books/NBK431054/.
4. Gonzales-Luna AJ, Carlson TJ, Garey KW. Gut microbiota changes associated with Clostridioides difficile infection and its various treatment strategies. Gut Microbes. 2023;15(1):2223345.
5. Stallhofer J, Steube A, Katzer K, Stallmach A. Microbiota-based therapeutics as new standard-of-care treatment for recurrent Clostridioides difficile infection. Visc Med. 2024;40(2):82-91.6. Aby ES, Vaughn BP, Enns EA, Rajasingham R. Cost-effectiveness of fecal microbiota transplantation for first recurrent Clostridioides difficile infection. Clin Infect Dis. 2022;75(9):1602-1609.
7. Johnson S, Lavergne V, Skinner AM, et al. Clinical practice guideline by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA): 2021 focused update guidelines on management of Clostridioides difficile infection in adults. Clin Infect Dis. 2021;73(5):e1029-e1044.
8. Wang Y, Hunt A, Danziger L, Drwiega EN. A comparison of currently available and investigational fecal microbiota transplant products for recurrent Clostridioides difficile infection. Antibiotics (Basel). 2024;13(5):436.9. Grigoryan Z, Shen MJ, Twardus SW, et al. Fecal microbiota transplantation: uses, questions, and ethics. Med Microecol. 2020;6:100027.
10. Davidovics Z. Fecal transplant. In Wyllie R, Hyams JR, Kay M, eds.: Pediatric Gastrointestinal and Liver Disease. 6th ed. Elsevier; 2021:1039-1042.
11. Barron M. Fecal microbiota transplants (FMT): past, present and future. ASM.org. 2024. https://asm.org/articles/2024/february/fecal-microbiota-transplants-past-present-future. Accessed August 8, 2024.
12. Gonzales-Luna AJ, Carlson TJ, Garey KW. Review article: safety of live biotherapeutic products used for the prevention of Clostridioides difficile infection recurrence. Clin Infect Dis. 2023;77(Suppl 6):S487-S496.13. Jolaiya T, Smith S. Evolving technologies in gastrointestinal microbiome era and their potential clinical applications. In: Pellicano R, Fagoonee S, Ajayi A, eds. 3Ts in Gastrointestinal Microbiome Era: Technology, Translational Research and Transplant. MDPI; 2021:4.
14. DuPont HL, DuPont AW, Tillotson GS. Microbiota restoration therapies for recurrent Clostridioides difficile infection reach an important new milestone. Therap Adv Gastroenterol. 2024;17:9.
15. McChalicher CW, Auniņš JG. Drugging the microbiome and bacterial live biotherapeutic consortium production. Curr Opin BIotechnol. 2022;78:102801.
16. Rebyota administration highlights: Rebyota administration—one dose. One complete microbiome-based biotherapeutic. June 5, 2024. www.rebyotahcp.com/why-rebyota/#rebyota-administration-highlights. Accessed October 3, 2024.17. Kao D, Wong K, Franz R, et al. The effect of a microbial ecosystem therapeutic (MET-2) on recurrent Clostridioides difficile infection: a phase 1, open-label, single-group trial. Lancet Gastroenterol Hepatol. 2021;6(4):282-291:8.
18. Haber SL, Raney CRK, Larson TL, Lau JP. Fecal microbiota transplantation for recurrent Clostridioides difficile infection. Am J Health Syst Pharm. 2019;76(13):935-942.
The content contained in this article is for informational purposes only. The content is not intended to be a substitute for professional advice. Reliance on any information provided in this article is solely at your own risk.
To comment on this article, contact rdavidson@uspharmacist.com.






