US Pharm. 2020;45(6):19-24.
ABSTRACT: Generic drug shortages have strained an already-burdened healthcare system. Unfortunately, drug shortages can ultimately lead to adverse patient outcomes and an extreme increase in the costs of addressing them. Healthcare professionals, especially pharmacists, are consistently tasked with the management of this public-health crisis. In order to appropriately address the problem, researchers have attempted to explore the root causes of generic drug shortages. Studies suggest that drug shortages are ultimately due to a broad range of factors. However, the most likely causes of generic drug shortages are economic and regulatory in nature.
The passage of the Hatch-Waxman Act of 1984 transformed the generic drug industry by incentivizing the development of generic medications, thereby increasing the affordability of medications and access to care.1,2 It is estimated that 90% of all filled prescriptions in the United States are generic drugs.2 In recent years, however, generic drug shortages have plagued the healthcare industry, disrupting patient care and emerging as a major public-health crisis.3
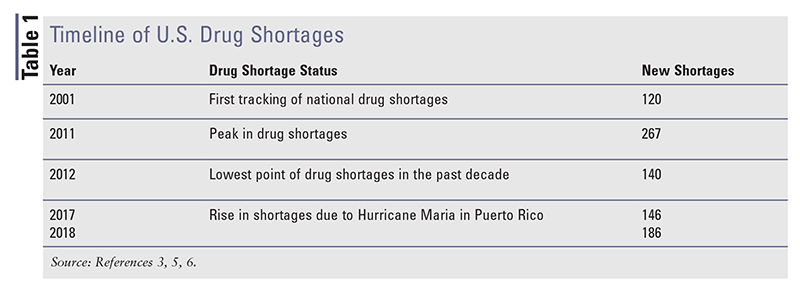
Drug shortages have become an area of intense study. The FDA defines drug shortages as “a period when the demand or projected demand for the drug within the United States exceeds its supply,” and the American Society of Health-System Pharmacists describes it as “a supply issue that affects how the pharmacy prepares or dispenses a drug product or influences patient care when prescribers must use an alternative agent.”3,4 Formal tracking of drug shortages began in 2001, and experts have seen marked fluctuations in drug shortages since then, with 2011 constituting the peak of the generic-drug-shortage crisis (TABLE 1).3,5,6
In an effort to counteract the detrimental effects of drug shortages, public-health officials, healthcare professionals, and economists have attempted to establish the root causes.3 At present, drug shortages are thought to result from a combination of factors, including low profitability, quality-control challenges, company mergers, complex generic-drug supply chains, natural disasters, and regulatory hurdles.3 Legislation such as the FDA Safety and Innovation Act (FDASIA) and its amendments, including the Generic Drug User Fee Amendments (GDUFA) I and II and Title X, has attempted to address drug shortages; although notable improvements have occurred, the crisis persists.3,7-9
Generic drug shortages affect many areas of the healthcare spectrum. Pharmacists often bear the brunt of the burden of handling drug shortages, such as coordinating medication procurement, developing alternative drug protocols, and addressing the dissatisfaction of patients and other members of the healthcare team.3,5,10 Without robust solutions to address drug shortages, global crises such as the coronavirus disease 2019 (COVID-19) pandemic further exacerbate the situation. Therefore, a better understanding of the origins of drug shortages and their impact on the healthcare industry and patient care is vital to the development of innovative solutions to address this public-health crisis.
Overall Impact
The unavailability of medications can have a number of deleterious effects. To address drug shortages, institutions and clinicians often develop processes or react in ways that best circumvent the problem.3 Unfortunately, there may not be optimal solutions, and shortages can lead to increased cost, reduced patient and staff satisfaction, and a negative effect on patient safety.3
It is estimated that health systems spend approximately $200 million in direct costs and between $216 million and $359 million in indirect costs to address drug shortages.3,5 Indirect costs are often incurred to support pharmacists, pharmacy technicians, and others who 1) procure medications through various modalities, including the gray market, where medications are sold at highly inflated prices, sometimes exceeding 3,000%; 2) ration available product; 3) determine alternative therapies and develop treatment protocols; 4) revise information-technology systems; 5) reschedule procedures; and 6) educate front-line staff on changes.3,5,11,12
Pharmacists are often tasked with revising their role to assist with managing drug shortages, which can negatively affect their professional satisfaction.3,12 Unfortunately, persons unaware of the root causes of drug shortages may erroneously blame pharmacy personnel, thereby damaging interprofessional relationships. In a 2017 Institute for Safe Medication Practices (ISMP) survey about drug shortages, 62% of pharmacy personnel indicated that medical, nursing, and hospital leadership staff, other staff, and patients were frequently or always frustrated with pharmacy personnel during drug shortages.10
Patient satisfaction is also negatively affected during drug shortages owing to the considerable financial and emotional burdens placed on patients to purchase costly alternative therapy or stop treatment if no alternatives exist.3,11 These changes can lead to delays in care, poor outcomes, disease progression, and increased anxiety, all of which reduce patient satisfaction.3
The ISMP receives numerous reports of harmful patient consequences during critical shortages.10 This may be due to deviation from the standard of care, delay in medication administration, and medication errors due to the provider’s lack of familiarity with alternative therapy.14 Drug shortages have touched all medical specialties, but the impact on infectious diseases, oncology, emergency and critical care, pain management, and nutrition is noteworthy.1,3
Specialties Subject to Drug Shortages
Infectious Diseases: Antibiotic shortages have persisted in recent years, leading to negative patient outcomes. A 2018 survey of infectious-diseases physicians noted that the top five antibiotic shortages involved injectable generics, forcing 75% of survey respondents to use broader-spectrum antibiotics and 45% to prescribe second-line therapy or less effective therapy.15 Seventy-three percent of respondents reported that these alterations negatively affected patient care or outcomes.15 A 2017 study found that hospitals experiencing a shortage of piperacillin-tazobactam had a 30% increase in risk of hospital-onset Clostridium difficile (P <.05) owing to alternative antibiotic usage.16
Oncology: Unlike infectious diseases, for which potential alternative agents often exist, there is a paucity of alternative agents for certain oncologic disorders.1 Notably, older generic injectable products are most likely implicated in shortages, and older injectable products are often used for pediatric cancers.3,11,17,18 Acute lymphoblastic leukemia (ALL), the most common childhood cancer, is curable in 90% of cases; however, 82% of medications used to treat ALL were in short supply between 2009 and 2019.3 The substitution of agents lacking sufficient evidence can lead to reduced efficacy, increased cost, poor drug tolerance, reduced quality of life, drug toxicity, and death.11
Emergency and Critical Care: Many far-ranging drug shortages have plagued the care of acutely ill patients. One study reported that a 2011 shortage of norepinephrine led to a 15% higher odds of in-hospital mortality in patients treated for septic shock.14 Shortages of epinephrine autoinjectors used to manage anaphylaxis in patients with severe allergies have led patients to use expired products, purchase medications at exorbitant prices, and resort to impractical delivery using syringes with vials.19
Pain Management: The shortage of opioids can lead to adverse patient outcomes. A study in JAMA Oncology demonstrated poor control of cancer pain during an opioid shortage.20 Also, reports submitted to the ISMP detailed fatal or near-fatal errors related to substitution of one opioid for another during drug shortages.21
Nutrition: In the past 10 years, many components of parenteral nutrition have been unobtainable, with the American Society for Parenteral and Enteral Nutrition noting that some clinicians have never practiced in a time without ingredient shortages.22 This is not optimal for the training of clinicians, and it can contribute to medication errors. Furthermore, the long-standing shortages of ingredients such as multivitamins have led to adverse patient outcomes.23
Root Causes of Generic Drug Shortages
It is clear that the impact of drug shortages is wide-ranging. Therefore, it is imperative that the root causes of drug shortages, particularly generic drug shortages, be better understood. Although the full range of causes has not yet been fully elucidated, economic forces are likely driving drug shortages.3
Low Profitability: Generic drugs are considered not lucrative because of the high cost of manufacturing these products relative to the gains.3,24 This is especially true of parenteral generic agents that require costly, specialized equipment to meet sterility and other requirements but have low profit margins.3,11 For this reason, entry into the generic market has declined and exit from the generic market has increased.24 Furthermore, competition in the generic market, including race-to-the-bottom pricing strategies, can disincentivize production.3 With a lack of incentive to produce these drugs, manufacturers may reduce their inventory of medications or completely remove drugs from the market, resulting in drug shortages.3 In order to reduce shortages of generic drugs, especially parenteral agents, it is important to offer manufacturers incentives to reliably supply these essential medications.
Quality: A loss of quality in production can lead to drug shortages.3 In order to maintain quality, especially in older generic drugs, expensive updates to equipment and facilities are required.3,25 Without reward and incentivization for sustained quality, it is unlikely that generics manufacturers will continue to supply critically important older generic drugs.26 Therefore, designing a merit system that is transparent to purchasers may also reward high-performing manufacturers and curb the drug shortage crisis.3
Mergers: A median of two manufacturers exists for each generic product.7 In this already-constricted market, consolidation of companies can further exacerbate drug shortages as the merged companies streamline inventory and improve profitability.3 Therefore, mergers often lead to a reduced quantity of medications, and—in the case of single-source products—their complete discontinuation.11 A reduction in the number of manufacturers can place stress on the remaining manufacturers to supply medications to meet the demand of clinicians and patients.3
Complex Supply Chains: The high cost of manufacturing drives manufacturers to participate in complex supply chains that can add pressure to an already precarious infrastructure.3 These manufacturers often rely on outside entities to provide active pharmaceutical ingredients (APIs) and finished dosage forms (FDFs) to help lower the cost of production and increase their profit margins.3,8 In fact, 88% of sites that manufacture APIs and 63% of those that manufacture FDFs are located abroad, with the majority in China and India.3 Although this strategy may lower costs, it can lead to complications in enforcement of quality and management of sensitive supply chains, which can in turn amplify the potential for drug shortages.3 Changes in geopolitical circumstances of foreign entities that produce APIs and FDFs impact the global supply chain, and the COVID-19 pandemic has exposed the vulnerabilities that such a strategy poses to drug availability in the U.S.27
Natural Disasters: Natural disasters can play a role in drug shortages.3,13 In 2017, the loss of electricity in Puerto Rico following Hurricane Maria led to suspended drug manufacturing and numerous drug shortages.3,28 Currently, there is concern that the COVID-19 pandemic will lead to an escalation in drug shortages as well.27
Regulatory Hurdles: Regulatory hurdles also play a role in generic drug shortages. It is speculated that the annual GDUFA I fees to manufacturers—implemented through FDASIA in 2012 to permit a more streamlined and transparent review process of abbreviated New Drug Applications (NDAs) and therefore generic medications—may have been cost prohibitive, leading manufacturers to exit the generic market.8,9,24 The FDA’s 2016 Unapproved Drug Initiative, which required completion of an NDA for older generic drugs that were on the market prior to establishment of the FDA, may have also deincentivized manufacturers from continuing with production.11 Also, when good manufacturing practices are not met by generic manufacturers, delays in feedback from the FDA can also lead to drug shortages.11 Attempts to mitigate drug shortages and incentivize generic drug manufacturers, such as Title X of FDASIA in 2012, required manufacturers to provide early notification of supply disruptions or discontinuations, and in 2017 GDUFA II attempted to reduce fees and economic barriers for manufacturers that have rectified the problem.8
Conclusion
Drug shortages can lead to a number of deleterious outcomes, including adverse patient outcomes, a rise in costs, and reduced patient and staff satisfaction.3 Generic drugs are especially impacted by shortages.24 The causes of drug shortages are multifold, but low profitability, loss of quality, company mergers, complex supply chains, natural disasters, and regulatory hurdles are thought to be implicated in a majority of cases.3 In order to successfully mitigate drug shortages, it is crucial that medications be produced reliably and at a cost that is equitable and sustainable to the manufacturer.3 Ultimately, the goal is to ensure the availability of products in order to best care for patients.
REFERENCES
1. Elzawawy AM, Kerr DJ. Variation in the availability of cancer drug generics in the United States of America. Ann Oncol. 2013;24(suppl 5):v17-v22.
2. FDA. Generic drugs. www.fda.gov/drugs/buying-using-medicine-safely/generic-drugs. Accessed May 2, 2020.
3. FDA. Report. Drug shortages: root causes and potential solutions. www.fda.gov/drugs/drug-shortages/report-drug-shortages-root-causes-and-potential-solutions. Accessed May 18, 2020.
4. Fox ER, Birt A, James KB, et al. ASHP guidelines on managing drug product shortages in hospitals and health systems. Am J Health Syst Pharm. 2009;66(15):1399-1406.
5. Fox ER, Sweet BV, Jensen V. Drug shortages: a complex health care crisis. Mayo Clin Proc. 2014;89(3):361-373.
6. American Society of Health-System Pharmacists. Drug shortages statistics. www.ashp.org/Drug-Shortages/Shortage-Resources/Drug-Shortages-Statistics. Accessed March 24, 2020.
7. Berndt ER, Conti RM, Murphy SJ. The Landscape of US Generic Prescription Drug Markets, 2004-2016. Working paper 23640. Cambridge, MA: National Bureau of Economic Research; 2017.
8. Berndt ER, Conti RM, Murphy SJ. The generic drug user fee amendments: an economic perspective. J Law Biosci. 2018;5(1):103-141.
9. FDA. Food and Drug Administration Safety and Innovation Act (FDASIA). www.fda.gov/regulatory-information/selected-amendments-fdc-act/food-and-drug-administration-safety-and-innovation-act-fdasia. Accessed March 24, 2020.
10. Institute for Safe Medication Practices. Drug shortages continue to compromise patient care. www.ismp.org/resources/drug-shortages-continue-compromise-patient-care. Accessed March 13, 2020.
11. Nonzee NJ, Luu TH. The drug shortage crisis in the United States: impact on cancer pharmaceutical safety. Cancer Treat Res. 2019;171:75-92.
12. Shaban H, Maurer C, Willborn RJ. Impact of drug shortages on patient safety and pharmacy operation costs. Fed Pract. 2018;35(1):24-31.
13. Phuong JM, Penm J, Chaar B, et al. The impacts of medication shortages on patient outcomes: a scoping review. PLoS One. 2019;14(5):e0215837.
14. Vail E, Gershengorn HB, Hua M, et al. Association between US norepinephrine shortage and mortality among patients with septic shock. JAMA. 2017;317(14):1433-1442.
15. Gundlapalli AV, Beekmann SE, Graham DR, et al. Antimicrobial agent shortages: the new norm for infectious diseases physicians. Open Forum Infect Dis. 2018;5(4):ofy068.
16. Gross AE, Johannes RS, Gupta V, et al. The effect of a piperacillin/tazobactam shortage on antimicrobial prescribing and Clostridium difficile risk in 88 US medical centers. Clin Infect Dis. 2017;65(4):613-618.
17. Alpert A, Jacobson M. Impact of oncology drug shortages on chemotherapy treatment. Clin Pharmacol Ther. 2019;106(2):415-421.
18. McBride A, Holle LM, Westendorf C, et al. National survey on the effect of oncology drug shortages on cancer care. Am J Health Syst Pharm. 2013;70(7):609-617.
19. The EpiPen shortage: how has it come to this? Lancet Child Adolesc Health. 2018;2(12):839.
20. Haider A, Qian Y, Lu Z, et al. Implications of the parenteral opioid shortage for prescription patterns and pain control among hospitalized patients with cancer referred to palliative care. JAMA Oncol. 2019;5(6):841-846.
21. Institute for Safe Medication Practices. A shortage of everything except errors: harm associated with drug shortages. www.ismp.org/resources/shortage-everything-except-errors-harm-associated-drug-shortages. Accessed April 1, 2020.
22. American Society for Parenteral and Enteral Nutrition. Product shortages. www.nutritioncare.org/ProductShortages. Accessed March 31, 2020.
23. Holcombe B, Andris DA, Brooks G, et al. Parenteral nutrition electrolyte/mineral product shortage considerations. JPEN J Parenter Enteral Nutr. 2011;35(4):434-436.
24. Conti RM, Berndt ER. Four facts concerning competition in US generic prescription drug markets. Int J Econ Bus. 2020;27(1):27-48.
25. Hernandez I, Hershey TB, Donohue JM. Drug shortages in the United States: are some prices too low? JAMA. 2020;323(9):819-820.
26. Woodcock J, Wosinska M. Economic and technological drivers of generic sterile injectable drug shortages. Clin Pharmacol Ther. 2013;93(2):170-176.
27. FDA. Coronavirus (COVID-19) update: FDA takes further steps to help mitigate supply interruptions of food and medical products. www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-takes-further-steps-help-mitigate-supply-interruptions-food-and. Accessed April 1, 2020.
28. Thomas K, Kaplan S. Hurricane damage in Puerto Rico leads to fears of drug shortages nationwide. www.nytimes.com/2017/10/04/health/puerto-rico-hurricane-maria-pharmaceutical-manufacturers.html. Accessed April 1, 2020.
The content contained in this article is for informational purposes only. The content is not intended to be a substitute for professional advice. Reliance on any information provided in this article is solely at your own risk.
To comment on this article, contact rdavidson@uspharmacist.com.






