US Pharm. 2021;46(6):58-59.
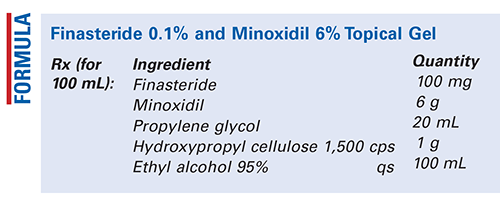
Method of Preparation: Calculate the required quantity of each ingredient for the total amount to be prepared. Accurately weigh or measure each ingredient. Combine the propylene glycol and 75 mL of the ethyl alcohol 95%; mix well, and carefully heat to 55°C to 60°C. Remove from heat and add the minoxidil and finasteride; stir until dissolved. After cooling, slowly add the hydroxypropyl cellulose to the rapidly stirred mixture and stir until gelling is complete. This may take a couple of hours. Add sufficient ethyl alcohol 95% to final volume and mix well. Package and label.
Use: This preparation has been used in the treatment of alopecia in men.
Packaging: Package in tight, light-resistant containers.
Labeling: Keep out of reach of children. Discard after ____ [time period]. For external use only. Keep tightly closed.
Stability: A beyond-use date of 30 days may be used for this preparation.1
Quality Control: Quality-control assessment can include theoretical weight compared with actual weight, pH, specific gravity, active drug assay, color, clarity, texture–surface, texture–spatula spread, appearance, feel, rheologic properties, physical observations, and preservative-effectiveness test.2,3
Discussion: Finasteride (Propecia, Proscar, C23H36N2O2, MW 372.54) occurs as a white to off-white, crystalline solid that is freely soluble in alcohol and very slightly soluble in water. Finasteride prevents the conversion of testosterone to dihydrotestosterone in the body and is for use in men only. The Propecia product is used to treat male pattern hair loss, and the Proscar product is used to treat symptoms of benign prostatic hyperplasia in men with an enlarged prostate. Proscar, if this is used as the drug source, is available as film-coated tablets that contain 5 mg of finasteride and the following inactive ingredients: hydrous lactose, microcrystalline cellulose, pregelatinized starch, sodium starch glycolate, hydroxypropyl cellulose LF, hydroxypropyl methylcellulose, titanium dioxide, magnesium stearate, talc, docusate sodium, FD&C Blue 2 Aluminum Lake, and yellow iron oxide.4
Minoxidil (Loniten, Rogaine, C9H15N5O, MW 209.25) is used as an antihypertensive and as a topical hair-growth stimulant. Minoxidil occurs as a white or off-white, odorless, crystalline solid that is slightly soluble in water to the extent of approximately 2 mg/mL; is readily soluble in propylene glycol or ethanol; and is almost insoluble in acetone, chloroform, or ethyl acetate. Minoxidil is commercially available as a 2% topical solution and a 5% foam, these having different presentations.1,5
Propylene glycol (C3H8O2) occurs as a clear, colorless, viscous, practically odorless liquid with a sweet taste, somewhat resembling glycerin. Propylene glycol has a specific gravity of 1.038 g/mL and is miscible with acetone, chloroform, 95% ethanol, glycerin, and water. Propylene glycol is not miscible with fixed oils or light mineral oil. It is stable and may be mixed with numerous other solvents.6
Hydroxypropyl cellulose is a white to slightly yellow-colored, odorless, tasteless powder. It is widely used in oral and topical pharmaceutical formulations. The pH of a 1% w/v aqueous solution is in the range of 5.0 to 8.5. Hydroxypropyl cellulose is soluble 1 in 2 parts water, 1 in 2.5 parts ethanol, and 1 in 5 parts propylene glycol, but it is practically insoluble in glycerin and oils.7
Ethyl alcohol (alcohol, ethanol, grain alcohol, C2H5OH, MW 46.07) is a clear, colorless, mobile, volatile liquid with a slight, characteristic odor and a burning taste. Its specific gravity is between 0.812 and 0.816, and its boiling point is 78.15°C. Ethyl alcohol is miscible with chloroform, glycerin, and water.8
REFERENCES
1. U.S. Pharmacopeia/National Formulary [current revision]. Rockville, MD: U.S. Pharmacopeial Convention, Inc; May 2021.
2. Allen LV Jr. Summary of quality-control testing for sterile and nonsterile compounded preparations, part 1: physical and chemical testing. IJPC. 2019;23(3):211-216.
3. Allen LV Jr. Summary of quality-control testing for sterile and nonsterile compounded preparations, part 2: microbiological testing. IJPC. 2019;23(4):299-303.
4. RxList. Proscar. www.rxlist.com/proscar-drug.htm#description. Accessed May 11, 2021.
5. Drugs.com. Minoxidil (topical). www.drugs.com/ppa/minoxidil-topical.html. Accessed May 11, 2021.
6. Quinn ME. Propylene glycol. In: Sheskey PJ, Hancock BC, Moss GP, Goldfarb DJ, eds. Handbook of Pharmaceutical Excipients. 9th ed. London, England: Pharmaceutical Press; 2021:867-870.
7. Fulzele SS, Fulzele S. Hydroxypropyl cellulose. In: Sheskey PJ, Hancock BC, Moss GP, Goldfarb DJ, eds. Handbook of Pharmaceutical Excipients. 9th ed. London, England: Pharmaceutical Press; 2021:515-521.
8. Quinn ME. Ethanol. In Sheskey PJ, Hancock BC, Moss GP, Goldfarb DJ. Handbook of Pharmaceutical Excipients. 9th ed. London, England: Pharmaceutical Press; 2021:404-407.
The content contained in this article is for informational purposes only. The content is not intended to be a substitute for professional advice. Reliance on any information provided in this article is solely at your own risk.
To comment on this article, contact rdavidson@uspharmacist.com.






