US Pharm. 2023;48(6):36-41.
ABSTRACT: Biological products, including biosimilars, have increasingly become available. Although biosimilars offer patients a safe, effective, and less expensive alternative to the reference/innovator product, a number of factors continue to limit their use in clinical practice. These include insufficient familiarity with biosimilars and their availability, concerns about their efficacy and safety, and lack of knowledge about their interchangeability. Pharmacists are well placed to foster awareness about biosimilars and make clinical recommendations that may increase their use in clinical practice. Pharmacists can give prescribers and patients crucial information about biosimilars, such as the manufacturing and approval processes and available efficacy and safety data. They can also dispel common misconceptions about biosimilars and educate prescribers and patients about the potential clinical and financial benefits associated with biosimilar use.
The increased availability of biological therapies has transformed the treatment, management, and prevention of a number of chronic medical conditions, including autoimmune diseases and cancer, by targeting and interfering with the physiological mechanisms that contribute to the underlying pathogenesis of the disease; however, the high costs associated with biologics remain a significant obstacle to patient access. The IQVIA Institute recently reported that over the past 5 years, the biologics market in the United States grew more rapidly than that for nonbiological therapies, with an average rate of 12.5% annually on an invoice-price basis, and that the biologics market accounted for 46% of spending.1 The IQVIA Institute’s report also noted that expanding the biosimilar market can enhance patients’ access to much-needed medications and has the potential to generate substantial savings for both the patient and the healthcare system.1
On March 6, 2015, the FDA approved the first biosimilar available in the U.S.2 This product, Zarxio (filgrastim-sndz), is a biosimilar to filgrastim (Neupogen; Amgen).2 According to the FDA, biosimilars are one of the fastest-growing areas of therapeutics, and their availability has improved patients’ access to biological therapies.3 The past few years have seen a considerable expansion of the biosimilar market for the treatment and prevention of chronic diseases in various areas of medicine, such as oncology, rheumatology, ophthalmology, endocrinology (diabetes), and nephrology, as well as for supportive care.
The FDA defines a biosimilar as a biological medication that is highly comparable but not identical to, and has no clinically meaningful differences from, an existing FDA-approved biologic, referred to as a reference or innovator product.3 Moreover, a biosimilar must have the same therapeutic indication, mechanism of action, route of administration, dosage, strength, and adverse-effect profile as its corresponding reference/innovator product.3 The biosimilar must also have comparable effectiveness, purity, and chemical and biological activity to the reference/innovator product.4 It is important to note that minor differences are likely to occur during the manufacturing process and that acceptable inconsequential differences in clinically inert ingredients can exist between a biosimilar and its reference/innovator product.4
According to the FDA and the Biosimilars Forum, biosimilar availability provides patients with more treatment options, increases access to biological products, and potentially lowers healthcare costs through competition.3,5 On average, biosimilars in the U.S. are at least 30% less expensive than their reference/innovator products.5 The Association for Accessible Medicines reported that in 2020 the use of biosimilars resulted in $7.9 billion in savings for the U.S. healthcare system.6,7
Although biosimilars have the capacity to reduce chronic disease–related healthcare costs for both the patient and the healthcare system, their integration into clinical practice faces challenges based on prescriber and patient concerns.4 For example, the accessibility of biosimilars and the growing number of biosimilars in development have created reluctance among some prescribers to switch from a biological agent because they think that the lower cost means that the biosimilar is of lesser quality.4,8 Other factors include prescriber discomfort over prescribing biosimilars instead of the reference/innovator products, concerns about interchangeability between biosimilars and their reference/innovator products, and patients’ concerns about efficacy and safety and the reluctance to switch from a biological product to a biosimilar.4 Payer considerations are another primary factor that may contribute to the low acceptance and use of biosimilars.4
A recent systematic review evaluated pharmacists’ knowledge of and insights into biosimilars to assess knowledge gaps.9 The researchers found that pharmacists’ knowledge and awareness about biosimilars varied greatly and generally were limited, specifically regarding critical concepts including interchangeability and switching, efficacy, safety, and indication extrapolation. Across studies, the percentage of pharmacists who were comfortable with biosimilars being prescribed for all of the indications previously used for the reference/innovator products ranged from 22% to 51%; the percentage of those who found it acceptable to switch from a reference/innovator product to a biosimilar ranged from 26% to 84%; and 22% to 74% of pharmacists reported having attended biosimilar training programs. The researchers concluded that if pharmacists were more knowledgeable about biosimilars, they would be better able to educate prescribers about them, which in turn could foster greater acceptance of their use.9
As the number of biosimilars continues to grow, pharmacists must be prepared to effectively counsel prescribers and patients on pertinent clinical information, mechanisms of action, administration, storage and dispensing, monitoring parameters, potential adverse effects, and regulation of biosimilars.
Biosimilars Basics
It is important to understand that biosimilars are not generics. To prove the agent’s biosimilarity and receive FDA approval, the manufacturer must include data from analytical studies, preclinical testing, and clinical trials (typically a comparative-design study or studies that are adequate to establish safety, purity, and potency).10 Additionally—in contrast to generics, which are generated by chemical synthesis and are exact copies of their reference drug—biosimilars are highly comparable to the reference drug with regard to structure, safety, efficacy, and immunogenicity but are not identical.11
Key facts about biosimilar products include the following3,12,13:
• A biosimilar is a biological product that has no clinically meaningful differences from a reference/innovator product.
• A biosimilar may be utilized in patients previously treated with the reference product and in those not previously treated with the reference product.
• Although a biosimilar is approved by the FDA via an abbreviated pathway, it undergoes rigorous evaluation to ensure that its safety and effectiveness are identical to those of the reference product. Biosimilar manufacturers adhere to the same quality assessments as their reference/innovator products.
• An interchangeable product is a biosimilar that meets additional FDA requirements. Depending on state pharmacy laws, a pharmacist may substitute an interchangeable for a reference product without consulting the prescriber. The FDA does not approve a product as interchangeable unless a company specifically seeks this determination.
To enhance access to biological medications, the Biologics Price Competition and Innovation Act of 2009 authorized the FDA to supervise an abbreviated and expedited pathway [i.e., 351(k) pathway] for the approval of biosimilars.10,12,14 This process reduces the time and cost of developing a biosimilar without compromising the safety and effectiveness of the product.14
Because the development of a biosimilar builds on what is already recognized and verified from clinical-trial data for the reference/innovator product, the procedure for developing a biosimilar centers on confirming that the biosimilar’s structure and function are highly similar to those of the reference product.14,15 The developmental process for biosimilars involves conducting clinical comparative-analysis trials in humans to confirm equivalent pharmacokinetics/pharmacodynamics and equivalent efficacy, safety, and immunogenicity as the reference/innovator product.12,15 See Reference 14 for more information on the approval process.
The Purple Book—the official database for all FDA-licensed biological products, including reference biologics, biosimilars, and interchangeable biosimilars—can serve as an excellent resource for pharmacists seeking to educate prescribers and patients about biosimilars. More information is available at https://purplebooksearch.fda.gov.
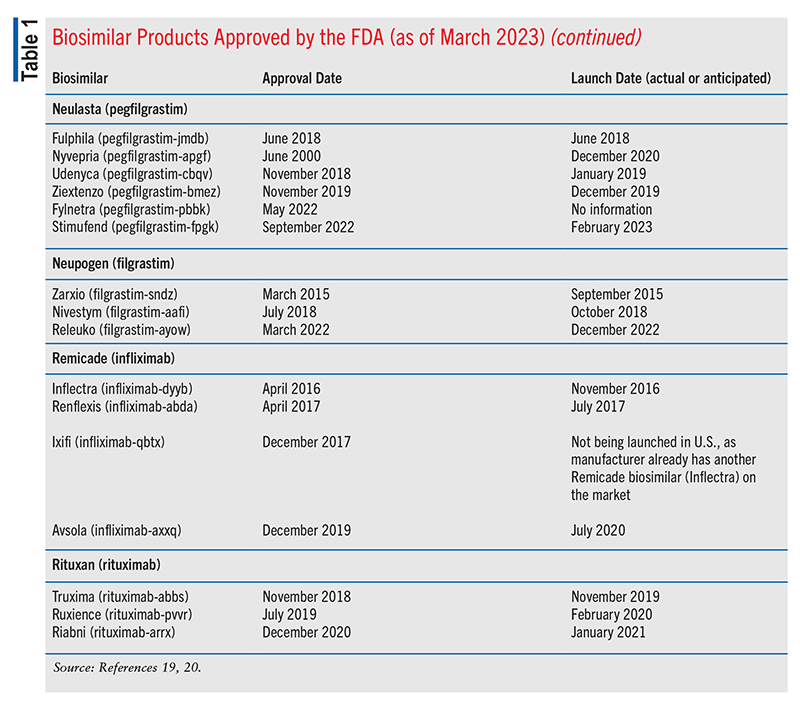
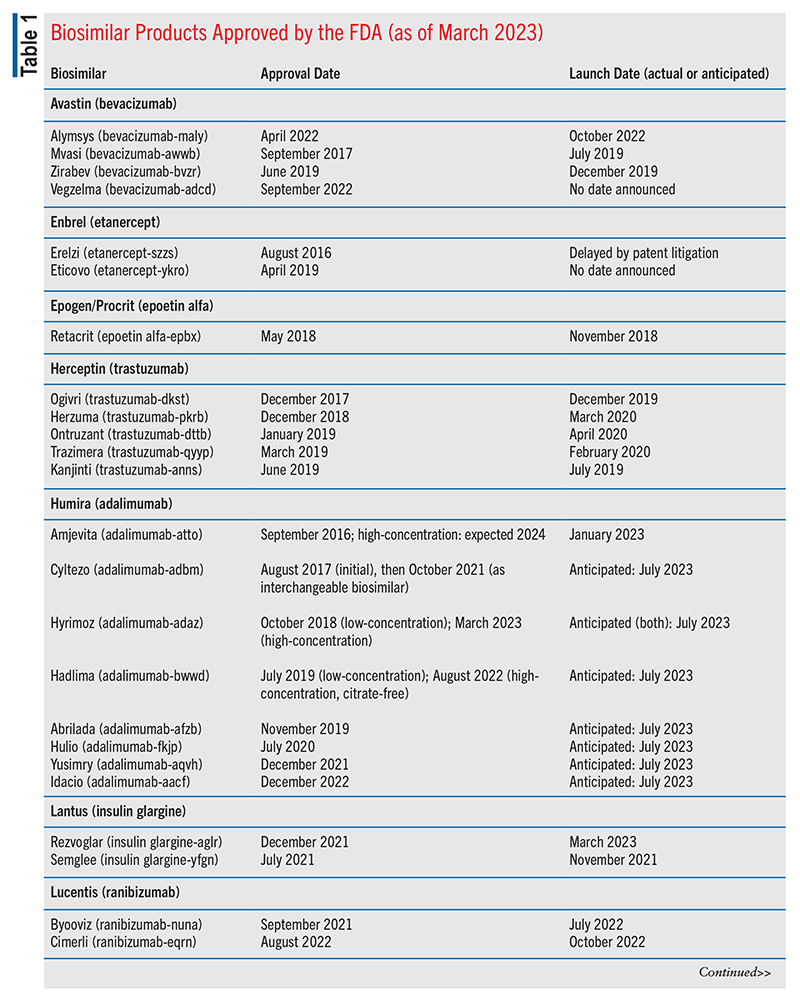
Cardinal Health’s 2023 Biosimilars Report on the 2022 biosimilars marketplace stated that of the 40 FDA-approved biosimilar products, 25 were available commercially (including four designated interchangeable and several others under consideration).16 The four interchangeable agents included two long-acting insulin biosimilars, one adalimumab biosimilar, and one ranibizumab biosimilar.16 (For context, because an interchangeable biosimilar is expected to produce the same clinical results as the reference/innovator product in any given patient, an interchangeable product may be substituted for the reference product, if needed.16-18) As of March 2023, 28 of the FDA-approved biosimilar products have been launched. See TABLE 1 for more information.19,20
Recent Developments
Following its approval by the FDA in September 2021, ranibizumab-nuna—the first biosimilar to an ophthalmologic product, Lucentis (ranibizumab)—was launched under the brand name Byooviz in July 2022. The second ophthalmologic biosimilar, Cimerli (ranibizumab-eqrn), was FDA-approved in August 2022 and launched in October 2022. Cimerli was also designated as interchangeable, making it the only predominantly medical-benefit biosimilar to achieve this designation to date.16
Amjevita (adalimumab-atto), the first biosimilar to Humira (adalimumab), became available in the U.S. in January 2023.21 This agent was approved by the FDA in September 2016.21
In March 2023, the FDA approved a citrate-free, high-concentration formulation of Hyrimoz (adalimumab-adaz) injection (100 mg/mL), a biosimilar comparable to its reference/innovator product, Humira (adalimumab).22 This biosimilar is approved to treat seven of the indications covered by Humira: rheumatoid arthritis, juvenile idiopathic arthritis, psoriatic arthritis, ankylosing spondylitis, Crohn’s disease, ulcerative colitis, and plaque psoriasis. The manufacturer plans to launch both the high-concentration formulation and the low-concentration formulation (approved in October 2018) of Hyrimoz in the U.S. on July 1, 2023.22
Cardinal Health’s 2023 Biosimilars Report noted that the patents of more than 50 biological agents will expire over the next decade, creating the opportunity for more biosimilars to enter the market, and that at least eight adalimumab biosimilars were expected launch in 2023.16 A recent Cardinal Health survey of 350 prescribers that focused on prescriber knowledge of biosimilars revealed some important insights. Among the participants (dermatologists, gastroenterologists, ophthalmologists, and rheumatologists), rheumatologists demonstrated ongoing and expanding knowledge about biosimilars, as 76% said that they were very familiar with biosimilars and 62% stated that they would be very comfortable with prescribing biosimilars. The degree of knowledge about biosimilars was even higher among gastroenterologists (81%), but rates were lower for ophthalmologists (33%) and dermatologists (31%). Among dermatologists, 82% strongly agreed or agreed that educating patients on the biosimilars’ safety and efficacy was a top priority, and 60% were somewhat comfortable with switching from one biosimilar product to another. Ninety-three percent of gastroenterologists were at least somewhat comfortable with prescribing adalimumab biosimilars, compared with 86% of rheumatologists and 75% of dermatologists.16
A cross-sectional study from 2022 investigated pharmacists’ confidence in educating patients about biosimilars and collected details about how they responded to common queries.23 Pharmacists’ primary concerns about biosimilars included lack of knowledge, low acceptance rates, and inferior efficacy and safety. Knowledge gaps about biosimilars included the inability to define biosimilars, provide patients with information about adverse drug reactions, or trust that biosimilars undergo the same testing process as biologics. Pharmacists were least confident about describing the procedures for biosimilar manufacturing and assessment. More educational initiatives are warranted to improve pharmacists’ biosimilar knowledge, which will enhance their counseling ability and possibly increase patients’ acceptance of these products.23
The Pharmacist’s Role in Biosimilars Education
Because of their drug expertise, pharmacists can be instrumental in educating prescribers and patients about the availability of biosimilars; providing clinical safety and efficacy data, as well as information about the approval process; and alleviating any existing concerns.
As the number of biosimilars on the market continues to grow, pharmacists can increase awareness about the safety and efficacy of biosimilars by keeping abreast of current clinical data. They can also implement effective educational strategies to dispel common misconceptions about the use of biosimilars and explain that the FDA requires biosimilars to undergo the same rigorous safety and effectiveness evaluations as the reference/innovator product. Pharmacists can also be instrumental in identifying patients who may be appropriate candidates for biosimilars and making clinical recommendations tailored to patient need.
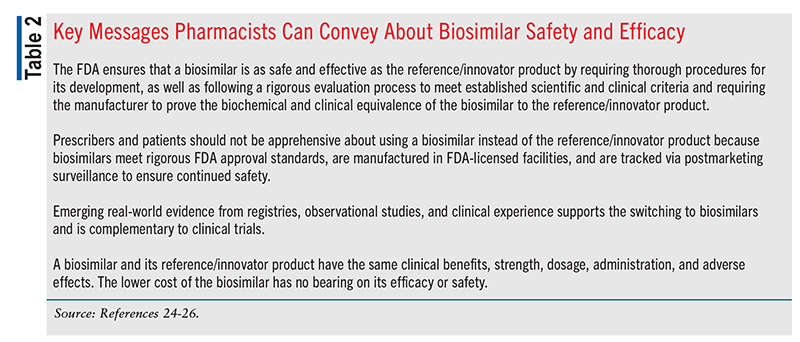
Pharmacists can also educate prescribers and patients about challenges and barriers that may limit biosimilar use—such as misunderstanding about interchangeability and substitution, lack of acceptance, and unawareness of potential cost savings—and address any questions or concerns.23 Addressing these barriers by assuring prescribers and patients about biosimilars’ comparable efficacy and safety may foster increased adoption of these products into clinical practice (TABLE 2).24-26 Pharmacists can also give prescribers and patients pertinent information about the clinical and economic benefits of biosimilars. Engaging prescribers and patients in conversations about biosimilars may help mitigate apprehension about the safety and effectiveness of biosimilars and promote confidence in their use; it may also lead to cost savings and greater access to these valuable products for the treatment, management, and prevention of various chronic diseases and result in improved clinical outcomes in those patients who need them.
Conclusion
The promising role of biosimilars continues to expand in several areas of therapeutics, and these products have the potential to improve clinical outcomes for many patients with chronic diseases. The availability of biosimilars may increase access to therapies that some patients might not otherwise be able to afford. The implementation of effective educational measures is crucial to enhancing the adoption of biosimilars into clinical practice, which in turn may increase clinician and patient confidence and result in health and financial benefits for patients and the healthcare system.
Pharmacists can inform prescribers and patients that the FDA’s well-established and superior practice standards for approval mean that a biosimilar or interchangeable biosimilar is just as effective and safe as the FDA-approved reference/innovator product. When counseling prescribers and patients about biosimilars, pharmacists should direct them to valuable resources such as the following:
• FDA—Biosimilars. www.fda.gov/drugs/therapeutic-biologics-applications-bla/biosimilars
• Biosimilars Council—Biosimilars Handbook. www.biosimilarshandbook.org
• American Pharmacists Association—Overview of Biologics and Biosimilar Products. www.pharmacist.com/Advocacy/Issues/Biosimilars
• FDA—Biosimilar Basics for Patients. www.fda.gov/drugs/biosimilars/biosimilar-basics-patients
Equipped with this knowledge, patients can make informed decisions about biosimilars, and they should be encouraged to discuss this option with their primary healthcare provider.
REFERENCES
1. Aitken M, Kleinrock M, Pritchett J. Biosimilars in the United States 2023-2027: Competition, Savings, and Sustainability. Parsippany, NJ: IQVIA Institute; January 2023.
2. Raedler LA. Zarxio (filgrastim-sndz): first biosimilar approved in the United States. Am Health Drug Benefits. 2016;9(Spec Feature):150-154.
3. FDA. Biosimilars. Overview for health care professionals. www.fda.gov/drugs/biosimilars/overview-health-care-professionals#What%20is%20a%20biosimilar%20product. Accessed March 31, 2023.
4. Okoro RN. Biosimilar medicines uptake: the role of the clinical pharmacist. Explor Res Clin Soc Pharm. 2021;1:100008.
5. Biosimilars Forum. Societal benefits of biosimilars. https://biosimilarsforum.org/biosimilars-101/societal-benefits-biosimilars. Accessed March 31, 2023.
6. Association for Accessible Medicines. Study finds U.S. generic and biosimilar savings totaled a record $338 billion in 2020. https://accessiblemeds.org/resources/press-releases/study-finds-us-generic-and-biosimilar-savings-totaled-record-338-billion. Accessed March 31, 2023.
7. American Pharmacists Association. Biosimilar basics for patients [brochure]. https://aphanet.pharmacist.com/sites/default/files/files/APhA_Biosimilar_Basics_for_Patients1.pdf. Accessed May 2, 2023.
8. Kay J, Schoels MM, Dörner T, et al. Consensus-based recommendations for the use of biosimilars to treat rheumatological diseases. Ann Rheum Dis. 2018;77(2):165-174.
9. Mohd Sani N, Aziz Z, Panickar R, Kamarulzaman A. Pharmacists’ perspectives of biosimilars: a systematic review. BioDrugs. 2022;36(4):489-508.
10. Markus R, Liu J, Ramchandani M, et al. Developing the totality of evidence for biosimilars: regulatory considerations and building confidence for the healthcare community. BioDrugs. 2017;31(3):175-187.
11. Triplitt C, Hinnen D, Valentine V. How similar are biosimilars? What do clinicians need to know about biosimilar and follow-on insulins? Clin Diabetes. 2017;35(4):209-216.
12. Biosimilars Forum. Biosimilars 101. https://biosimilarsforum.org/biosimilars-101. Accessed March 31, 2023.
13. Kozlowski S, Woodcock J, Midthun K, Sherman RB. Developing the nation’s biosimilars program. N Engl J Med. 2011;365(5):385-388.
14. FDA. Biosimilars. Review and approval. www.fda.gov/drugs/biosimilars/review-and-approval. Accessed March 31, 2023.
15. Biosimilars Council. Biosimilars handbook: how are biosimilars developed and made? www.biosimilarshandbook.org/medical-professional-learning-track/how-are-biosimilars-developed-and-made-mp. Accessed March 31, 2023.
16. Cardinal Health. 2023 biosimilars report. www.cardinalhealth.com/content/dam/corp/web/documents/Report/cardinal-health-biosimilars-report-2023.pdf. Accessed March 31, 2023.
17. FDA Center for Drug Evaluation and Research and Center for Biologics Evaluation and Research. Considerations in Demonstrating Interchangeability With a Reference Product: Guidance for Industry. Silver Spring, MD: FDA Center for Drug Evaluation and Research; May 2019.
18. Arnet I, Verbeek M, Almarsdóttir AB, et al. Community pharmacists’ preparedness for substituting biologics and dispensing biosimilars—lessons learned from a multinational survey. Explor Res Clin Soc Pharm. 2021;4:100084.
19. Biosimilars Council. FDA-approved biosimilars. https://biosimilarscouncil.org/resource/fda-approved-biosimilars. Accessed March 31, 2023.
20. The Center for Biosimilars. Biosimilar approvals. www.centerforbiosimilars.com/biosimilar-approvals. Accessed March 31, 2023.
21. PRN Newswire. Amjevita™ (adalimumab-atto), first biosimilar to Humira®, now available in the United States. www.prnewswire.com/news-releases/amjevita-adalimumab-atto-first-biosimilar-to-humira-now-available-in-the-united-states-301734177.html. Accessed March 31, 2023.
22. Novartis. Sandoz receives US FDA approval for biosimilar Hyrimoz® (adalimumab-adaz) high-concentration formulation. www.novartis.com/news/media-releases/sandoz-receives-us-fda-approval-biosimilar-hyrimoz-adalimumab-adaz-high-concentration-formulation. Accessed March 31, 2023.
23. Gasteiger C, Gasteiger N, Petrie KJ. Pharmacists’ confidence in explaining biosimilars to patients before a nationwide medicine change: a cross-sectional study. Explor Res Clin Soc Pharm. 2022;8:100199.
24. Jacobs I, Singh E, Sewell KL, et al. Patient attitudes and understanding about biosimilars: an international cross-sectional survey. Patient Prefer Adherence. 2016;10:937-948.
25. Allocati E, Godman B, Gobbi M, et al. Switching among biosimilars: a review of clinical evidence. Front Pharmacol. 2022;13:917814.
26. Edwards CJ, Hercogová J, Albrand H, Amiot A. Switching to biosimilars: current perspectives in immune-mediated inflammatory diseases. Expert Opin Biol Ther. 2019;19(10):1001-1014.
The content contained in this article is for informational purposes only. The content is not intended to be a substitute for professional advice. Reliance on any information provided in this article is solely at your own risk.
To comment on this article, contact rdavidson@uspharmacist.com.