US Pharm. 2019;44(11):33-36.
According to the International Classification of Headache Disorders, 3rd edition (ICHD-3), cluster headache (CH) is a primary headache that is a type of trigeminal autonomic cephalalgia (TAC).1 CH is known as such because the attacks occur in cluster cycles in which an affected person may experience recurrent attacks at a rate of one to eight episodes per day for weeks or months at a time.1 Although the syndrome was publicly documented, categorized, and discussed over 60 years ago, much information is still unknown about this disorder, as only a few large-scale clinical studies have been conducted to assess potential treatment options for those affected by CH.2
A lack of clinical evidence for preventive and acute treatment, combined with the high severity of debilitating pain, led to the disorder being termed a suicide headache.3-7 From misdiagnoses to inadequate treatment options, many sufferers experience extreme reductions in quality of life, which leads to higher incidences of depressive disorders, sleep disturbances, anxiety, drug usage, suicidal ideation, and suicide.3,8 According to a 2017 retrospective analysis conducted to assess the characteristics, demographics, and treatment patterns, those who suffered from CH were two to three times more likely to present with one of the aforementioned psychological disorders.3
Epidemiology
With an estimated lifetime prevalence of approximately 124 per 100,000 adults worldwide and an estimated prevalence of 500,000 in the United States, CH may not be as rare as clinicians originally assumed.8,9 Although disease onset may occur at any age, CH onset generally occurs before age 30 years in 71% of patients.8 Males are approximately three times more likely than females to experience cluster headaches.1,4,10 In approximately 42% of patients, a proper diagnosis may take 5 years or longer from symptom onset, as the disorder is frequently misdiagnosed as migraine, sinusitis, or allergies.8
Diagnosis
Previously referred to as histaminic cephalalgia, Horton’s headache, vidian neuralgia, Sluder’s neuralgia, or hemicrania angioparalytic, CH is classified in the ICHD-3 by the International Headache Society as the occurrence of at least five severe headache attacks that present as strictly unilateral pain. Headaches may be orbital, supraorbital, temporal, or any combination. Additionally, associated pain may last from 15 minutes to 3 hours and can occur from once every other day up to eight times per day.1 As a TAC, the pain is associated with at least one of the following autonomic symptoms: lacrimation, ipsilateral conjunctival injection, miosis, ptosis, nasal congestion, facial sweating, eyelid edema, and/or rhinorrhea.1 Lastly, attacks may not be caused by any other disorder nor by another ICHD-3 diagnosis.1
Management
Current management of CH is similar to the treatment of migraine and consists of nonpharmacologic and pharmacologic methods. Both approaches should be integrated into the management of CH so optimal symptom relief can be achieved. Nonpharmacologic measures include managing or avoiding triggers, smoking and alcohol cessation, and lifestyle modifications.11
Pharmacologic treatment of CH can be abortive or prophylactic. Abortive therapy treats acute attacks while prophylactic treatment prevents attacks from occurring. Prophylactic treatment is usually started simultaneously with abortive treatment to induce remission and is subsequently tapered.11 An example is the concomitant use of the selective serotonin agonists sumatriptan and zolmitriptan with ergotamine. Ergot alkaloids can increase the potential side effects of serotonin receptor agonists and should not be used within 24 hours of each other, according to their package inserts.12,13 Serotonin receptor agonists also need to be hepatically dosed.12,13 Patients who take verapamil should have electrocardiographic monitoring due to its potential to prolong the PR interval.11 For prophylactic treatment, verapamil is the first-line or preferred agent.11 Valproic acid, topiramate, and ergotamine have limited evidence.11
The mainstay of acute treatment of cluster headaches is selective serotonin receptor agonists and high-flow oxygen. Medications and their doses used for abortive and prophylactic treatment of cluster headaches can be found in TABLES 1 and 2, respectively.

Device
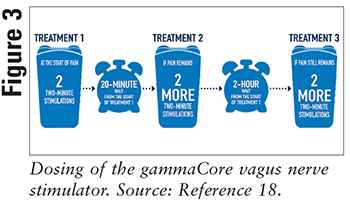
Designed by electroCore, gammaCore (FIGURE 1) is a noninvasive vagus nerve stimulator (nVNS) that has been approved for adjunctive use in prophylactic and acute treatment of cluster and migraine headaches in adult patients.14 By stimulating the vagus nerve, gammaCore can activate the parasympathetic system, which will inhibit vagal afferents to the trigeminal nucleus caudalis and result in a reduction of pain.15 The device produces a series of 5-kilohertz sine waves every 40 milliseconds.16 It can deliver a maximum of 40 volts and 60 milliamps output current when applied to the neck, as seen in FIGURE 2.17 The amplitude of stimulation is user controlled, which allows for personalized therapy. Dosing or recommended use of the gammaCore device can be seen in FIGURE 3.18


The approximate cost of gammaCore is $598 per month, according to electroCore’s customer support team, and can only be purchased, refilled, reloaded, or recharged with a prescription. Each charge is estimated to last approximately 31 days.
Adverse effects found in clinical trials include application-site reactions, muscu-loskeletal disorders, dysgeusia, metallic taste, oropharyngeal pain, dizziness, and naso-pharyngitis. None of these adverse effects were found to be clinically significant.
Contraindications include having a metallic device such as a stent, bone plate, or bone screw implanted at or near the neck or using another device at the same time such as a transcut-aneous electrical nerve stimulation unit, muscle stimulator, or mobile phone. Patients should consult with their physician to determine if the gammaCore device is an appropriate treatment option. Additional safety information can be found in the package insert.
Efficacy
Several clinical trials have been published that test the efficacy of nVNS. Popular clinical trials included the Prevention and Acute Treatment of Chronic Cluster Headache (PREVA), Acute Treatment of Cluster Headache (ACT1), the extension from ACT1 (ACT2), and Prospective Study of Non-Invasive Vagus Stimulation for the Acute Treatment of Migraine (PRESTO). The clinical trials took place in several countries, including Germany, the United Kingdom, Belgium, the U.S., and Italy. The population in all trials included patients over age 18 years. PREVA, ACT1, and ACT2 tested the efficacy in patients diagnosed with CH, while PRESTO focused on patients diagnosed with migraine. The PREVA trial compared standard of care (SoC) plus nVNS or SoC alone, whereas the others utilized nVNS and sham (placebo) with or without SoC.19 The primary endpoint of the PREVA trial was the mean reduction of CH attacks per week, which resulted in an average of 3.9 fewer attacks per week (95% CI: 0.5, 7.2; P = 0.02).19
Other clinical trials monitored pain associated with CH attacks or migraines. Higher response rates and pain-free status were noted in the nVNS groups when compared with sham.15,20,21 The ACT1 and ACT2 trials featured subgroups of episodic CH (eCH) and chronic CH (cCH), which showed that nVNS has more efficacy in patients with eCH when compared with cCH.15,20 In summary, the clinical trials have proven the efficacy of nVNS in CH and migraine.
Conclusion
The gammaCore device is indicated for the acute treatment of pain in CH and migraine headaches in adults. It is not proven to be effective in the prophylactic treatment of CH or migraine and was not developed to eliminate SoC treatments. The gammaCore nVNS was designed to be used in conjunction to improve patients’ quality of life. However, this device could be an option for patients interested in first exploring nonpharmacologic treatments. Further trials are needed to compare the efficacy without SoC treatment. New technology almost always comes with a high cost. As such, gammaCore may not be an affordable option for most patients, but it may be worth the cost to some. A cost-effectiveness analysis might provide more insight to patients and prescribers. Overall, gammaCore has proven efficacious as a treatment option for CH and migraine. More information can be found at gammacore.com.
REFERENCES
1. Headache Classification Committee of the International Headache Society (IHS). The International Classification of Headache Disorders. 3rd ed. Cephalalgia. 2018;38(1):1-211.
2. Horton BT. Histaminic cephalgia. Lancet. 1952;72(2):92-98.
3. Choong CK, Ford JH, Nyhuis AW, et al. Clinical characteristics and treatment patterns among patients diagnosed with cluster headache in U.S. Healthcare Claims Data. Headache. 2017;57(9):1359-1374.
4. Fletcher J. Why cluster headaches are called “suicide headaches.” J Neurology Stroke. 2015;3(3)1-4.
5. Nesbitt AD, Goadsby PJ. Cluster headache. BMJ. 2012;344:e2407.
6. Mwamburi M, Liebler EJ, Tenaglia AT. Cost-effectiveness of gammaCore (non-invasive vagus nerve stimulation) for acute treatment of episodic cluster headache. Am J Manag Care. 2017;23(16 Suppl):S300-S306.
7. Sanchez del Rio M, Leira R, Pozo-Rosich P, et al. Errors in recognition and management are still frequent in patients with cluster headache. Eur Neurol. 2014;72(3-4):209-212.
8. Rozen TD, Fishman RS. Cluster headache in the United States of America: demographics, clinical characteristics, triggers, suicidality, and personal burden. Headache. 2012;52(1):99-113.
9. Russell MB. Epidemiology and genetics of cluster headache. Lancet Neurol. 2004;3(5):279-283.
10. Manzoni GC. Gender ratio of cluster headache over the years: a possible role of changes in lifestyle. Cephalalgia. 1998;18(3):138-142.
11. Weaver-Agostoni J. Cluster headache. Am Fam Physician. 2013;88(2):122-128.
12. Zomig [package insert]. Hayward, CA: Impax Pharmaceuticals; 2012.
13. Imitrex [package insert]. Research Triangle Park, NC: GlaxoSmithKline; 2017.
14. Medgadget. gammaCore personal non-invasive VNS cleared for episodic cluster headaches. www.medgadget.com/2017/04/gammacore-personal-non-invasive-vagus-nerve-stimulator-now-cleared-u-s-episodic-cluster-headaches.html. Accessed August 5, 2019.
15. Silberstein SD, Mechtler LL, Kudrow DB, et al. Non-invasive vagus nerve stimulation for the acute treatment of cluster headache: findings from the randomized, double-blind, sham-controlled ACT1 study. Headache. 2016;56:1317-1332.
16. Simon B, Blake J. Mechanism of action of non-invasive cervical vagus nerve stimulation for the treatment of primary headaches. Am J Manag Care. 2017;23(17 Suppl):S312-S316.
17. electroCore. gammaCore. www.electrocore.com. Updated June 2019. Accessed August 4, 2019.
18. Migraine dosing. gammaCore. www.gammacore.com/for-migraine/migraine-dosing. Updated June 2019. Accessed August 4, 2019.
19. Gaul C, Diener HC, Silver N, et al. Non-invasive vagus nerve stimulation for PREVention and Acute treatment of chronic cluster headache (PREVA): a randomised controlled study. Cephalalgia. 2016;36(6):534-546.
20. Goadsby PJ, de Coo IF, Silver N, et al. Non-invasive vagus nerve stimulation for the acute treatment of episodic and chronic cluster headache: a randomized, double-blind, sham-controlled ACT2 study. Cephalalgia. 2018;38(5):959-969.
21. Tassorelli C, Grazzi L, de Tommaso M, et al. Noninvasive vagus nerve stimulation as acute therapy for migraine: the randomized PRESTO study. Neurology. 2018;91:e364-e373.
To comment on this article, contact rdavidson@uspharmacist.com.






