US Pharm.2021;46(1)28-32.
Glaucoma is a relatively common ophthalmologic disease impacting the adult population. It is a major public health concern as a leading cause of irreversible vision loss. The progressive degeneration of retinal ganglionic cells results in increased intraocular pressure (IOP), which causes the irreversible blindness. Nearly 80 million people worldwide have the disease, with this number expected to increase to 112 million by 2040. In the United States, more than 3 million Americans aged 40 years and older are affected by it, accounting for up to 12% of all cases of blindness. Since glaucoma can remain asymptomatic for so long, many people do not realize they have it until the disease worsens; progressive visual-field loss is peripheral and usually asymmetric, allowing for compensation of the affected eye to correct any visual disturbances.1-3 This is why ophthalmologic examinations are very important.
Currently there is no cure for glaucoma, and lifelong treatment is usually required to manage this condition. There are different classes of medications used to help decrease IOP, but adherence to such therapies is often lacking, with a range anywhere from 5% to 80% across studies. Pharmacists are ideally situated to remind patients about the importance of routine eye exams and to assess and help improve adherence to glaucoma medications, decreasing glaucoma-related blindness.4
Definition and Classification
Glaucoma refers to a group of ophthalmologic disorders characterized by optic neuropathies and loss of retinal ganglion cells, leading to severe visual-field loss and, ultimately, blindness. The two most common forms of glaucoma include primary open-angle glaucoma (POAG) and primary angle-closure glaucoma (PACG). POAG is the predominant subtype, affecting almost 2.7 million Americans.3,5
In POAG, the angle of the eye between the iris and cornea remains open; however, aqueous flow through the drainage structure of the eye, called the trabecular meshwork, and uveoscleral routes is diminished, which can ultimately result in an increase in IOP. This increase in pressure can potentially cause mechanical stress and strain on the posterior structures of the eye and can also lead to changes in blood flow to the optic nerve, decreasing oxygen and nutrient supply. All of this can lead to retinal ganglion-cell death. However, almost half of patients with POAG have an IOP that is considered to be within normal range (12-22 mm Hg) at diagnosis, emphasizing the importance of not just relying on IOP measurements as the sole diagnostic tool. In the presence of normal IOP, this condition has been referred to as normal-pressure glaucoma. PACG occurs when there is partial blockage or complete closure of the trabecular meshwork by the peripheral iris, leading to increased IOP and optic-nerve damage.2,6,7
Chronic glaucoma, both POAG and PACG, is mainly asymptomatic. Patients do not usually suffer visual-field defects until approximately 30% of retinal ganglion cells have been lost; noticeable sight loss may take many years to develop. Vision loss due to glaucoma is described most often as the loss of peripheral vision. Patients may also complain about blurriness, dimness, or cloudiness.
Patients with PACG may experience an acute event that is characterized by well-marked symptoms including sudden and possibly painful vision loss, unilateral blurred vision, and halos around lights. These patients may also experience pain around the eye that could be accompanied by nausea and vomiting. Acute PACG is considered a medical emergency and requires laser iridotomy, a procedure that uses a laser to make a hole in the iris to allow more effective drainage from the eye.2,6-8
Risk Factors
There are a number of risk factors that increase a person’s chance of getting glaucoma. People older than age 60 years are six times more likely to get glaucoma than are younger people. Patients of African American, Asian, and Native American descent are more likely than Caucasians to develop the disease. Patients who have a family history of glaucoma are more likely to develop it later in life. Other comorbid conditions such as diabetes, heart disease, and hypertension can cause glaucoma, especially if the conditions are poorly managed. Physical injuries to the eye can cause trauma and thereby damage the optic nerve, leading to glaucoma. Other eye conditions can make glaucoma more likely to occur. Eye inflammation, retinal detachment, and eye tumors can potentially be other risk factors. Medications, such as corticosteroids, can also increase IOP, potentially leading to glaucoma. Table 1 lists some of the more common risk factors that make certain people more prone to developing the disease.7,9-11
Diagnosis and Screening
An accurate diagnosis of glaucoma requires an ophthalmologic exam that is beyond what is routinely performed in a primary care setting. Additional tests, such as measurement of IOP (tonometry), measurement of central corneal thickness (pachymetry), stereoscopic optic nerve examination (ophthalmoscopy), anterior angle chamber examination (gonioscopy), and formal visual field testing (perimetry) are required. Because visual-field changes may take years before they occur, the mainstay of detection of glaucoma is examination of the optic nerve head and retinal nerve fiber layer. These changes can be identified during the ophthalmoscopic examination. The importance of ophthalmologic examinations and early detection of glaucoma cannot be overstated, especially in those patients at risk for glaucoma.2,6,10,11
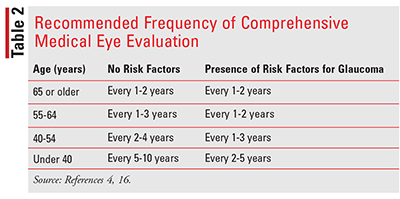
Screening of the general population for glaucoma is not recommended. However, the American Academy of Ophthalmology recommends that all patients older than age 40 years be evaluated and receive a baseline eye examination by an ophthalmologist or optometrist if they have not received one previously. Age and the presence of risk factors will determine the frequency of follow-up examinations. Pharmacists can play an important role in identifying risk factors and referring patients to eye-care specialists for appropriate screening and evaluation. Table 2 describes the recommended frequency for comprehensive eye examinations based on age and glaucoma-risk factors.12
Management
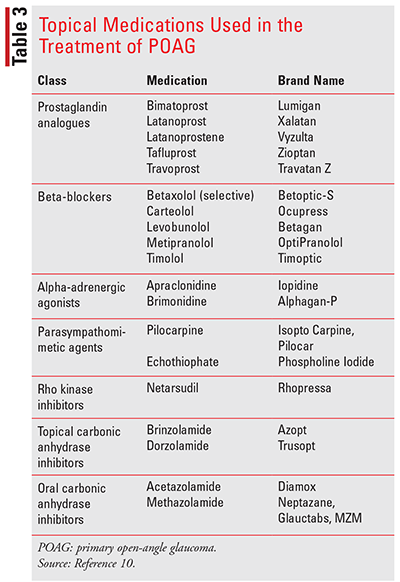
Although the prospect of permanent vision loss due to glaucoma is very scary to many patients, early detection and treatment can help reduce the chances of this occurring. When caring for patients with glaucoma, the goals of therapy include lowering IOP, preventing further damage to the optic nerve, and preventing further visual-function loss while increasing the patient’s quality of life with minimal adverse effects from treatment. Currently, there are several classes of medications that can be used for the management of POAG (Table 3). Prostaglandin analogs are considered first-line therapy; they effectively reduce IOP by up to 30% by improving uveoscleral outflow, lack systemic side effects, and require once-daily administration. Although systemic side effects are rare, patients may experience transient ocular effects, including blurred vision, itching, burning, or stinging, and excessive tearing. Other common side effects are quite different from other ophthalmic agents and therefore require patient education. The prostaglandin analogues may lengthen eyelashes, cause hyperpigmentation of the iris—especially in those with light-colored eyes—and cause hyperpigmentation of lids and periorbital skin. The hyperpigmentation of the iris appears to be permanent, but eyelid and eyelash changes are usually reversible upon discontinuation. These agents should be used with caution in patients with active intraocular inflammation or in those with a history of intraocular inflammation.2,10,13-17
Ophthalmic beta-blockers are an alternative to the prostaglandin analogues. This class of medications reduces IOP by decreasing aqueous humor production. Common side effects include burning or stinging in the eyes, inflammation, and blurred vision. Although approved for twice-daily dosing, these agents are more commonly dosed once daily; twice-daily dosing appears to provide no additional benefit when compared with once daily in the morning and may likely increase the risk of systemic events. Unlike the prostaglandin analogues, this class of medication has the potential to cause systemic effects, especially in patients with pulmonary, cardiac, or metabolic disorders. Systemic effects include hypotension, bradycardia, and bronchospasms. Betaxolol, which is beta selective, may minimize the systemic effects but is associated with reduced lowering of IOP when compared with the nonselective agents. Closing the eyes and practicing nasolacrimal occlusion following administration of the medication can help minimize systemic effects by limiting systemic absorption through the lacrimal ducts.2,10,17
Other medications that are used in the management of glaucoma include alpha-2 adrenergic agonists, topical and oral carbonic anhydrase inhibitors (CAIs), cholinergic agents, and rho kinase inhibitors. Alpha-2 adrenergic agonists lower IOP by decreasing aqueous humor production and increasing uveoscleral outflow. Although the efficacy of alpha-2 adrenergic agonists is similar to beta-blockers, their side-effect profile limits their use. Allergic conjunctivitis is common with these agents and is a cause for discontinuation in many patients. Other common ocular side effects include stinging, conjunctival hyperemia, foreign-body sensation, and dry eyes. Systemic side effects include hypertension, dry mouth, fatigue, dizziness, and mental confusion.
CAIs reduce aqueous humor production and are available as both oral and topical formulations. Oral CAIs are more effective compared with the topical formulations; however, their use is limited due to their significant systemic effects, including metabolic acidosis, paresthesias, bone-marrow suppression, and renal calculi. Common side effects of topical agents include taste disturbances, blurred vision, and eye discomfort. Although CAIs are sulfonamide agents, topical agents have been well tolerated in most patients with sulfonamide allergies.
Cholinergic agents lower IOP by increasing aqueous outflow through the trabecular meshwork. Once considered first-line agents, their use has declined over the years due to their unfavorable side-effect profile, and they are now considered third-line agents in the treatment of glaucoma. They cause contraction of the ciliary muscle, which leads to an increase of aqueous humor outflow. Local and systemic adverse effects are associated with their use. Locally they can cause miosis, which decreases visual acuity at night, a paradoxical increase in IOP, and retinal detachment. Systemic effects, although rare, include sweating, nausea, headache, increased salivation, and changes in blood pressure.2,10,17
Rho kinase inhibitors are the newest class of agents to be approved for the management of POAG. This class of medications decreases the resistance in the trabecular network, increasing outflow. Their efficacy appears to be similar to ophthalmic beta-blockers and may be used in combination with other antiglaucoma agents. The most frequent adverse effects are conjunctival hyperemia, conjunctival hemorrhages and cornea verticillate. However, there appears to be a lack of systemic effects, making this class of medication useful in patients with comorbidities.2,10,17
Role of the Pharmacist
Patient adherence to glaucoma medication has been shown to be poor. Almost half of patients stop using their medication after 6 months. Nonadherence to glaucoma treatments can contribute significantly to irreversible blindness in many patients. Studies have identified a variety of barriers associated with poor adherence. These include low self-efficacy, forgetfulness, and difficulty with eye drop administration, especially in older patients. Pharmacists can use communication techniques to help determine if patients understand their disease state and how to properly use their medications. Patients should be counseled about making their providers aware of their concerns, whether it is about cost, side effects, or difficulties with the prescribed regimen. Pharmacists can educate patients about how to properly administer eye drops and about common side effects, and they can address questions pertaining to their treatment regimen. Pharmacists are the medication experts and can help patients see the importance of adherence to their medications by explaining the benefits of the treatment. They can help patients develop ways to remember to use their eye drops and remind them, if they are using multiple drops of the same medication or are instilling more than one medication, to space the administration by at least 5 minutes. Overall, the impact a pharmacist can make on a patient’s life just by these small interventions can be life-changing. These counseling points and educational tips can help patients get the treatment they need and prevent irreversible blindness.4,18,19
Conclusion
Glaucoma is a complex and dangerous disease that develops over time and, if left untreated, will lead to irreversible vision loss. It is therefore difficult to diagnose early in one’s life. The good thing is that there are medications that can prevent the progression of the disease. Pharmacists play an important role in educating patients about the medications and the benefits of drug therapy. Patients may be wary of starting the treatment regimens, but counseling can help patients feel more comfortable. As the most accessible healthcare providers, pharmacists provide counseling that is beneficial and can help motivate patients to be adherent to their medications. Glaucoma may have no cure, but it is treatable. Pharmacists can help with patient adherence and drug management in order to prevent the detrimental effects of glaucoma.
Why should I be concerned about glaucoma?
Glaucoma is a fairly common disease and, if not treated early, can lead to permanent blindness. Often, patients are diagnosed later in life, when damage has already been done to the optic nerve that may be irreversible. Glaucoma can be prevented with proper medication use and avoidance of certain medications that can increase intraocular pressure.
Who is at risk of getting glaucoma?
People older than age 60 years are at increased risk of getting glaucoma. It is recommended that patients in this age group get regular eye exams. African Americans are more likely to develop the disease than Caucasians. Certain medical conditions such as diabetes and hypertension also increase the risk of developing the disease. Trauma and physical injury to the eyes definitely can damage the optic nerve, resulting in glaucoma. Corticosteroid use for prolonged duration puts patients at an increased risk for glaucoma.
What are some symptoms of glaucoma?
The symptoms can be so subtle that it is difficult to know one is developing the disease. It causes no pain, and vision is normal in the beginning. Gradually, a person with glaucoma will start to lose peripheral vision. Patients with known risk factors should be assessed by ophthalmologists regularly. Blurred vision, appearance of halos, and pain and redness of the eyes are all signs of an acute glaucoma attack. This is a medical emergency where vision loss can occur quickly. If you experience this, see your ophthalmologist or optometrist immediately; if that is not possible, go to the emergency room.
Do I need to be screened for glaucoma if I do not have any of the symptoms?
Screening is recommended for people who have certain risk factors. Some of these risk factors include being over the age of 40 years, having a family history of glaucoma, being diabetic, having high blood pressure, and being of African or Hispanic descent. The exams are not dangerous, and diagnosing the disease early would help in treating the disease and preventing it from having a big impact on vision.
Can glaucoma be treated?
Although there is no cure, there are many options to help manage glaucoma. The most common and often first treatment option is using medicated eye drops that try to lower the pressure in the eye. This will help prevent nerve damage and stop vision loss. It is important to remember to use the eye drops every day. Some cases may require laser treatment or surgery. Talk with your eye doctor about the best treatment for you.
Where can I find more information about glaucoma?
You can visit the National Institutes of Health, National Eye Institute website: www.nei.nih.gov/learn-about-eye-health/eye-conditions-and-diseases/glaucoma or the American Academy of Ophthalmology “What is Glaucoma?” website: www.aao.org/eye-health/diseases/what-is-glaucoma.
REFERENCES
1. Tham Y-C, Li X, Wong TY, et al. Global prevalence of glaucoma and projections of glaucoma burden through 2040: a systematic review and meta-analysis. Ophthalmology. 2014;121(11):2081-2090.
2. Weinreb RN, Aung T, Medeiros FA. The pathophysiology and treatment of glaucoma. JAMA. 2014;311(18):1901-1911.
3. BrightFocus Foundation. Glaucoma: facts & figures. Published July 6, 2015. www.brightfocus.org/glaucoma/article/glaucoma-facts-figures. Accessed December 8, 2020.
4. Dreer LE, Girkin C, Mansberger SL. Determinants of medication adherence to topical glaucoma therapy. J Glaucoma. 2012;21(4):234-240.
5. American Academy of Ophthalmology. Eye disease statistics. www.aao.org/eye-disease-statistics. Accessed December 8, 2020.
6. Jonas JB, Aung T, Bourne RR, et al. Glaucoma. Lancet. 2017;390(10108):2183-2193.
7. Greco A, Rizzo MI, De Virgilio A, et al. Emerging concepts in glaucoma and review of the literature. Am J Med. 2016;129(9):1000.e7-1000.e13.
8. Hu CX, Zangalli C, Hsieh M, et al. What do patients with glaucoma see? Visual symptoms reported by patients with glaucoma. Am J Med Sci. 2014;348(5):403-409.
9. McMonnies CW. Glaucoma history and risk factors. J Optom. 2017;10(2):71-78.
10. Gedde SJ, Vinod K, Wright MM, et al. Primary open-angle glaucoma preferred practice pattern. Ophthalmology. 2021;128(1):P71-P150.
11. Gedde SJ, Chen PP, Muir KW, et al. Primary angle-closure disease preferred practice pattern. Ophthalmology. 2021;128(1):P30-P70.
12. Chuck RS, Dunn SP, Flaxel CJ, et al. Comprehensive adult medical eye evaluation preferred practice pattern. Ophthalmology. 2021;128(1):P1-P29.
13. Xalatan (latanoprost) prescribing information. Puurs, Belgium: Pfizer Manufacturing Belgium NV; September 2020.
14. Travatan Z (travoprost) prescribing information. East Hanover, NJ: Novartis Pharmaceuticals Corporation; May 2020.
15. Lumigan (bimatoprost) prescribing information. Madison NJ: Allergan USA; September, 2020.
16. Zioptan (tafluprost)prescribing information. Lake Forest IL: Oak Pharmaceuticals; November 2018.
17. Marquis RE, Whitson JT. Management of glaucoma: focus on pharmacological therapy. Drugs Aging. 2005;22(1):1-21.
18. Newman-Casey PA, Robin AL, Blachley T, et al. Most common barriers to glaucoma medication adherence: a cross-sectional survey. Ophthalmology. 2015;122(7):1308-1316.
19. Feehan M, Munger MA, Cooper DK, et al. Adherence to glaucoma medications over 12 months in two us community pharmacy chains. J Clin Med. 2016;5(9):79.
To comment on this article, contact rdavidson@uspharmacist.com.