US Pharm. 2016;41(8):36-40.
ABSTRACT: Benign prostatic hyperplasia (BPH) is a common disorder in men with an incidence that increases with age. BPH often requires therapy when patients begin to experience lower urinary tract symptoms that affect quality of life. Current management strategies involve lifestyle modifications, pharmacotherapy, phytotherapy, and surgical interventions as indicated. Pharmacists are in the unique position of being accessible sources of healthcare information for the BPH patient population. Understanding the symptoms of this disorder and therapy options will be beneficial for pharmacists who have increased chances to answer BPH-related questions from their patients.
Benign prostatic hyperplasia (BPH) is a common disorder that presents in men and increases in incidence with age. It is characterized by the nonmalignant growth of the prostate gland that occurs in most men >40 years of age. The prevalence of BPH, as seen in several autopsy studies around the world, is estimated to be approximately 20% for men in their 40s, up to 60% for men in their 60s, and up to 90% for men in their 70s and 80s.1 Although almost all men will develop histologic or microscopic evidence of BPH by their eighth decade of life, the condition does not require treatment until it becomes symptomatic.
PATHOPHYSIOLOGY
Prostatic enlargement with increased age is correlated with smooth muscle hyperplasia and may eventually lead to bladder outlet obstruction (BOO).2 The most common presentation of subjective BPH-associated symptoms is lower urinary tract symptoms (LUTS).3 LUTS may further be classified into obstructive and irritative symptoms. Examples of obstructive symptoms include urine hesitancy, straining, weak flow, prolonged voiding, partial or complete urinary retention, and urinary incontinence. Common irritative symptoms include urinary frequency, urgency, nocturia, dysuria, and decreased void volume.1
DIAGNOSIS
Diagnosis of BPH often rules out other clinical manifestations that may present with similar symptoms. Examples include prostate cancer, prostatitis, bladder cancer, bladder stones, overactive bladder (OAB), interstitial cystitis, and urinary tract infections; all of which may also cause LUTS.3 Although symptoms related to BPH are often not life-threatening, they can be debilitating and affect quality of life (QOL) significantly. Thus, it is important to identify and correctly diagnose BPH in order to pursue an effective treatment strategy.
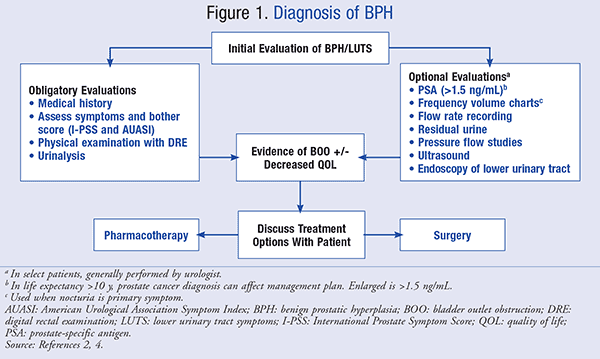
The American Urological Association (AUA) guideline panel made several recommendations for the diagnosis of BPH that were consistent with an article published by Abrams et al in 2009.2,4 The recommendations state that a basic evaluation should be performed on patients who are experiencing negative changes in their QOL due to LUTS. This evaluation may include several components, which are summarized in FIGURE 1. If the initial evaluation shows the presence of LUTS associated with one or more of the digital rectal examination (DRE) findings suspicious of prostate cancer, hematuria, abnormal prostate-specific antigen (PSA), pain, recurrent infection (infection should be assessed before referral), palpable bladder, or neurologic disease, the patient should be referred to a urologist for additional evaluation before pursuing treatment.4
CURRENT TREATMENT GUIDELINES
The goal of treatment is to relieve LUTS and slow the clinical progression of BPH while improving patient QOL. The 2010 updated treatment guideline from the AUA recommend that if the patient presents with LUTS, with or without uncomplicated prostate enlargement, and the symptoms are not affecting his QOL, then no further evaluation or treatment is recommended. The patient should be reassured and scheduled for a follow-up appointment with his physician if necessary.2 SIDEBAR 1 presents a case report of a patient with BPH.
Sidebar 1. BPH Case Report
Chief Complaint: LF is a 64-year-old white male presenting with fatigue and sleep pattern changes that have lasted over the past several years. He believes his poor sleep habits are due to frequent nighttime urination, occurring three to four times per night. He denies drinking any fluids in the evening. LF also complains of difficulty starting a stream, especially in public restrooms, and notes that this stream has become weaker over time. He also feels that it takes him longer to completely empty his bladder. He admits to “going a little in his shorts” after urinating and confirmed postvoid dribbling. He experiences some mild urgency but denies dysuria. He also confirms knowledge of all of the bathroom locations in his office building.
Past Medical History: LF denies blood in his urine, a past history of STDs, or other UTIs. He reluctantly reports difficulty keeping firm erections during sexual intercourse compared to when he was younger. However, he maintains that this issue is not one of his major concerns.
Family History: Negative family history of prostate cancer
Social History: Nonsmoker, no illicit drugs, mild alcohol consumption (2-3 beers per week)
Physical Examination: Male pattern baldness
Vital Signs: WNL
Genital Examination:
• Circumcised penis without lesions
• Two, nontender, normal-sized descended testicles
Digital Rectal Examination: Normal sphincter tone and hemoccult negative stool
• Negative for masses in the rectal vault except for an enlarged, firm prostate without nodules or tenderness
• Prostate size estimated to be 35 mL (normal size 20-25 mL)
Labs:
• Total cholesterol: 230 (LDL: 165, HDL: 45, TG: 100)
• PSA: 2.34 ng/mL (normal 0.0-4.0 ng/mL)
• TSH: WNL
• CMP/CBC: WNL
Prescription Medications: None
Nonprescription Medications:
• Niacin 1,000 mg 1 daily
• Cinnamon tablet 1 daily
Treatment: Therapy options were first discussed with LF, which included observation with lifestyle modifications and pharmacotherapy. Due to its invasive nature, surgical interventions should be discussed when there has been a failure of the less invasive treatments. Since LF’s presentation was not suggestive of prostate cancer (e.g., an elevated PSA >4.0 or the presence of nodules on the prostate during the examination), a biopsy of the prostate was not indicated. LF was started on finasteride 5 mg once daily. After about 2 months, he reported improved urinary flow, less nocturia, and an improved sleep pattern with less daytime fatigue. His only side effects were a slightly diminished libido and an occasional retrograde ejaculation.
Discussion: Finasteride was indicated in LF due to its benefit of decreasing prostate size while improving maximum urinary flow rate and associated LUTS. In addition, it is also indicated for male pattern baldness. Alpha-blockers could also be considered in this patient, with the added benefit of a quicker response by dilating the prostatic urethra, although with the possible result of a higher prevalence of retrograde ejaculation. Another option discussed with LF was the use of tadalafil. However, he did not express significant interest regarding the need for improvement of erectile dysfunction. If LF does not respond to the currently prescribed therapy, urodynamic studies could be ordered, which would evaluate the actual flow kinetics of the urine through the prostate and also the amount of residual urine in the bladder after micturition. The results of these tests could then lead to a referral to a urologist in anticipation of one of the various surgical procedures such as TURP.
BPH: benign prostatic hyperplasia; CMP/CBC: complete metabolic profile/complete blood count; HDL: high-density lipoprotein; LDL: low-density lipoprotein; PSA: prostate-specific antigen; STD: sexually transmitted disease; TG: triglyceride; TSH: thyroid-stimulating hormone; TURP: transurethral resection of the prostate; UTI: urinary tract infection; WNL: within normal limits.
Watchful Waiting
Watchful waiting or active surveillance is recommended for men who begin experiencing mild symptoms related to BPH. The physician and patient should discuss potential treatment options, including benefits and risks associated with each alternative, and identify a choice treatment based on shared decision-making.2 Additional optional evaluations could also be pursued at this time. In general, watchful waiting is appropriate in patients who are experiencing some symptoms that have not yet begun to affect their daily life.2
Lifestyle Modifications
If patients present with bothersome LUTS that begin affecting their QOL, lifestyle modifications should be recommended initially. Common lifestyle modifications and behavior recommendations include nightly fluid restriction, timed bladder voiding, double-voiding techniques, regular physical activity, treatment of constipation, and avoiding caffeine, alcohol, and highly seasoned or irritative foods.5 These recommendations help to improve symptoms and prevent progression of symptoms to the point of requiring pharmacotherapy or surgery.
Pharmacotherapy
If lifestyle modifications are insufficient in improving QOL, then pharmacotherapy may be indicated in patients who do not have absolute indications warranting surgery.5 Current oral pharmacotherapy options for managing BPH include alpha-adrenergic antagonists (alpha-blockers), 5-alpha-reductase inhibitors (5ARIs), muscarinic receptor antagonists (MRAs), and phosphodiesterase 5 (PDE5) inhibitors.6 A summary of the available agents in each class can be found in TABLE 1.5,6
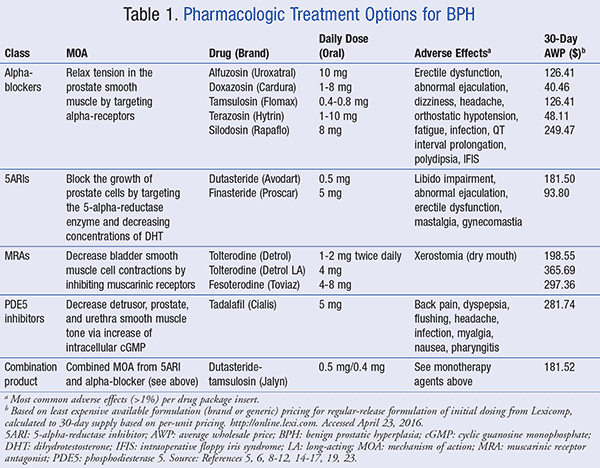
Alpha-Blockers: This class of medications is indicated for the treatment of patients with moderate-to-severe symptomatic BPH regardless of prostate size.5 Alpha-blockers work by blocking the alpha-adrenoceptors on the smooth muscle of the prostate, prostatic urethra, and bladder neck, leading to decreased muscle tone and reduction in bladder obstruction.5 All available alpha-blockers have comparable efficacy when given at appropriate doses and can help to improve urinary flow rate after a few hours or days after administration. The most common class-related adverse events are summarized in TABLE 1.5,6
In particular, intraoperative floppy iris syndrome (IFIS; i.e., poor pupil dilation and sudden constriction) is an ocular adverse event that may occur in those undergoing cataract surgery.5 Alpha-blocker treatment should be discontinued prior to cataract surgery and restarted once surgery is complete.5 Orthostatic hypo-tension with dizziness may also occur due to vasodilation induced by blockage of alpha-adrenergic receptors. Thus, caution should be exercised in those taking antihypertensive agents or with cardiovascular comorbidities. Vasodilatory effects are more commonly seen with doxazosin and terazosin, and are less common with alfuzosin, tamsulosin, and silodosin.7
Patients should be counseled to take alfuzosin, tamsulosin, and silodosin with or immediately after the same meal each day, and to swallow the capsule whole without crushing, chewing, or opening the contents.8-10 Tamsulosin capsule contents may be mixed with a small amount of acidic fruit juice or soft food when necessary, while silodosin should only be mixed with applesauce.9,10 Doxazosin and terazosin can be taken without regard to meals. In addition, patients should be cautious when stretching or moving suddenly from a sitting to a standing position in order to avoid orthostatic hypotension.11,12
5ARIs: These medications (dutasteride, finasteride) are also recommended for patients with moderate-to-severe symptomatic BPH in addition to an enlarged prostate. An enlarged prostate is defined by gland size >25 mL and/or PSA levels >1.5 ng/mL based on clinical trials.2 5ARIs have also been shown to decrease both serum dihydrotestosterone (DHT) and PSA levels, improving maximum urinary flow rate and LUTS with no difference in clinical efficacy between agents.2,13
A main difference between the two agents is the serum half-life for each, which is 3 to 16 hours for finasteride and 5 weeks for dutasteride.14,15 This may have implications on medication adherence and persistence of adverse effects, which may last well after the discontinuation of the drug.13 Another difference is that finasteride is indicated for male pattern hair loss (androgenetic alopecia), while for dutasteride, it is an off-label use. Both finasteride and dutasteride can be administered with or without food.14,15 In addition, dutasteride capsules must be swallowed whole to avoid irritation of the oropharyngeal mucosa.15
MRAs: These agents (tolterodine, fesoterodine) are recommended for patients experiencing BPH with OAB symptoms. Symptoms may include irritative LUTS such as urinary urgency, with or without urge incontinence, often with frequency and nocturia.2 MRAs were studied in clinical trials as add-on therapy with alpha-blockers or 5ARIs in cases of OAB associated with BPH. Both tolterodine and fesoterodine demonstrated a significant improvement in storage symptoms, with dry mouth being the most common side effect.16,17 Patients should be counseled to take these agents without regard to meals and swallow the formulations whole.16,17
PDE5 Inhibitors: There has been growing interest in the use of PDE5 inhibitors alone or in combination with previously mentioned therapies in men experiencing LUTS, regardless of preexisting erectile dysfunction (ED).18 Although the most current update of the AUA guideline for BPH management does not mention treatment with PDE5 inhibitors, the 2013 European Association of Urology (EAU) guidelines report that these agents (i.e., tadalafil, sildenafil, and vardenafil) can be utilized to quickly decrease urinary symptoms and also improve ED.7,18 Tadalafil currently has a labeled indication for BPH and should be administered around the same time each day without regard to meals.19
Combination Therapy: Also available on the market is a fixed-dose combination product of dutasteride (5ARI) plus tamsulosin (alpha-blocker), which is approved for the symptomatic management of BPH.20 Clinical trials have shown that the synergistic effect of the dual mechanisms of action is significantly superior to tamsulosin and dutasteride monotherapy in symptom improvement as well as reducing BPH clinical progression in men with enlarged prostates.20 Adverse events were consistent with those seen in monotherapy; however, the frequency of these events was shown to be higher. The AUA guideline recommends the use of this combination therapy in men who have moderate-to-severe symptomatic BPH, an enlarged prostate, and reduced urine flow rate and are at risk for disease progression.7,20
Recently, there has also been evidence for the combination of PDE5 inhibitors with 5ARI therapy. One study found that tadalafil (PDE5 inhibitor) combined with finasteride (5ARI) led to an improvement in LUTS associated with BPH, regardless of the presence of ED symptoms.21 However, there is currently no drug combination product containing both medications available on the market in the U.S.
Phytotherapy: Plant-based or herbal medications have also been used for those experiencing mild-to-moderate LUTS.22 Clinical trials have shown efficacy in the treatment of LUTS; however, many products are not standardized and long-term safety data are not always available.22 Although a number of clinical trials of these products are ongoing, the most updated AUA guideline currently does not recommend the use of phytotherapy or other alternative medicines for the management of LUTS secondary to BPH.2 Commonly seen therapy options may include Serenoa repens (saw palmetto), Pygeum africanum (tree bark), Cucurbita pepo (squash), and Urtica dioica (stinging nettle).2,22 It is important to weigh the risks and possible benefits of utilizing alternative treatment.
Surgical Treatment
The 2010 AUA guideline states that surgical intervention is appropriate for individuals with moderate-to-severe LUTS, acute urinary retention, or other complications due to BPH.2 Surgery is the most invasive BPH management strategy. Patients usually fail lifestyle modifications and pharmacotherapy management before proceeding to surgery. Patients may elect to pursue surgery as primary treatment, but the physician and patient should discuss the risks versus benefits and weigh other options.
The AUA guideline recognizes transurethral resection of the prostate (TURP), which involves boring a larger passageway through the center of the prostate allowing for better flow of urine, as the benchmark therapy among the surgical options. In addition, an open prostatectomy may be reserved for men with very enlarged prostate glands (volume >80-100 mL), bladder diverticula, or bladder stones.2
PHARMACIST’S ROLE
Pharmacists have the training required to provide expert advice and recommendations for medication therapy. Pharmacists in the community setting, in particular, are an accessible source of healthcare information to the inquiring BPH patient population (SIDEBAR 2). It is important for pharmacists to understand the terminology and symptoms associated with BPH in order to engage in knowledgeable discussions with patients regarding therapy options. In addition, counseling for BPH medications should include a review of risks and possible adverse effects, which are summarized in TABLE 1. Overall, BPH is a common disease state in the aging male population, and pharmacists play an important role in management due to the increased chances of encountering related questions from the community.
REFERENCES
1. Roehrborn CG. Benign prostatic hyperplasia: an overview. Rev Urol. 2005;7(suppl 9):S3-S14.
2. McVary KT, Roehrborn CG, Avins AL, et al. American Urological Association Guideline: Management of Benign Prostatic Hyperplasia (BPH). Linthicum, MD: American Urological Association; 2010:1-62, Appendix 278-285. www.auanet.org/education/guidelines/benign-prostatic-hyperplasia.cfm. Accessed March 14, 2016.
3. Lepor H. Pathophysiology, epidemiology, and natural history of benign prostatic hyperplasia. Rev Urol. 2004;6(suppl 9):S3-S10.
4. Abrams P, Chapple C, Khoury S, et al. Evaluation and treatment of lower urinary tract symptoms in older men. J Urol. 2009;181(4):1779-1787.
5. Fonseca J, Martins da silva C. The diagnosis and treatment of lower urinary tract symptoms due to benign prostatic hyperplasia with α-blockers: focus on silodosin. Clin Drug Investig. 2015;35(suppl 1):7-18.
6. Wang X, Wang X, Li S, et al. Comparative effectiveness of oral drug therapies for lower urinary tract symptoms due to benign prostatic hyperplasia: a systematic review and network meta-analysis. PLoS One. 2014;9(9):e107593.
7. Gratzke C, Bachmann A, Descazeaud A, et al. EAU guidelines on the management of non-neurogenic male lower urinary tract symptoms (LUTS), including benign prostatic obstruction. Eur Urol. 2015;67(6):1099-1109.
8. Uroxatral (alfuzosin) package insert. Cary, NC: Covis Pharmaceuticals, Inc; September 2013.
9. Flomax (tamsulosin) package insert. Ridgefield, CT: Boehringer Ingelheim Pharmaceuticals, Inc; October 2014.
10. Rapaflo (silodosin) package insert. Corona, CA: Watson Pharmaceuticals, Inc; January 2013.
11. Cardura (doxazosin) package insert. New York, NY: Pfizer; October 2001.
12. Hytrin (terazosin) package insert. North Chicago, IL: Abbott Laboratories; July 2009.
13. Carrasquillo RJ, Nealy SW, Wang DS. 5-Alpha-reductase inhibitors in diseases of the prostate. Curr Opin Endocrinol Diabetes Obes. 2014;21(6):488-492.
14. Proscar (finasteride) package insert. Whitehouse Station, NJ: Merck & Co, Inc; January 2014.
15. Avodart (dutasteride) package insert. Research Triangle Park, NC: GlaxoSmithKline; October 2011.
16. Detrol (tolterodine tartrate) package insert. New York, NY: Pharmacia & Upjohn Co, division of Pfizer; February 2011.
17. Toviaz (fesoterodine fumarate) package insert. New York, NY: Pfizer, Inc; August 2012.
18. Gacci M, Carini M, Salvi M, et al. Management of benign prostatic hyperplasia: role of phosphodiesterase-5 inhibitors. Drugs Aging. 2014;31(6):425-439.
19. Cialis (tadalafil) package insert. Indianapolis, IN: Lilly ICOS, LLC; October 2011.
20. Dimitropoulos K, Gravas S. Fixed-dose combination therapy with dutasteride and tamsulosin in the management of benign prostatic hyperplasia. Ther Adv Urol. 2016;8(1):19-28.
21. Glina S, Roehrborn CG, Esen A, et al. Sexual function in men with lower urinary tract symptoms and prostatic enlargement secondary to benign prostatic hyperplasia: results of a 6-month, randomized, double-blind, placebo-controlled study of tadalafil coadministered with finasteride. J Sex Med. 2015;12(1):129-138.
22. Allkanjari O, Vitalone A. What do we know about phytotherapy of benign prostatic hyperplasia? Life Sci. 2015;126:42-56.
23. Jalyn (dutasteride and tamsulosin) capsule package insert. Research Triangle Park, NC: GlaxoSmithKline; January 2015.
To comment on this article, contact rdavidson@uspharmacist.com.






